Associated Effects of Cadmium and Copper Alter the Heavy Metals Uptake by Melissa Officinalis
Abstract
:1. Introduction
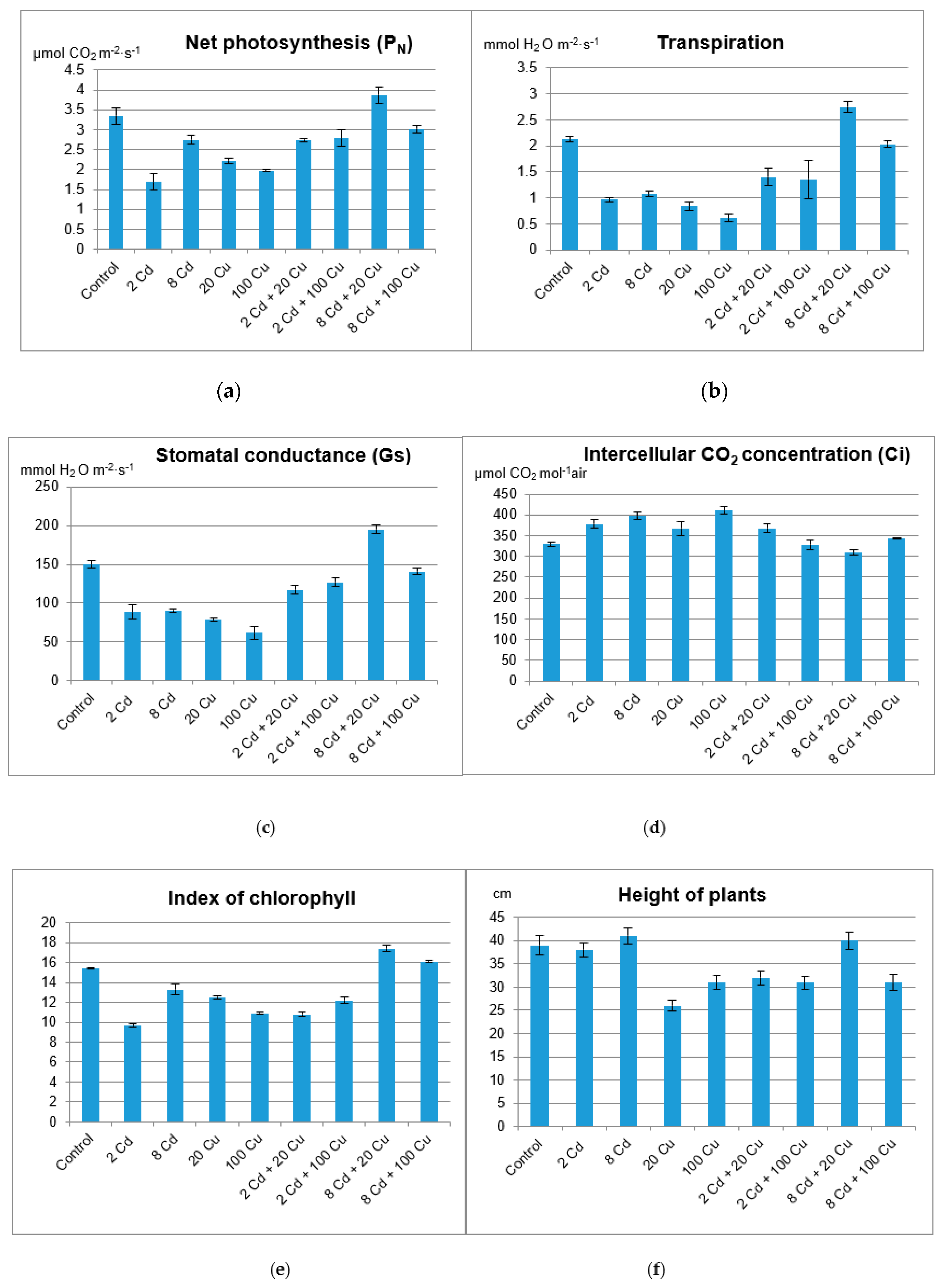
2. Results and Discussion
3. Materials and Methods
3.1. Soil Analysis
3.2. Preparation of Plant Material
3.3. Determination of Metals in Basil
3.4. Melissa Plant Growth and Its Physiological Activity
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bernal, M.; Ramiro, M.V.; Cases, R.; Picorel, R.; Yruela, I. Excess copper effect on growth, chloroplast ultrastructure, oxygen-evolution activity and chlorophyll fluorescence in Glycine max cell suspensions. Physiol. Plant. 2006, 127, 312–325. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 2018, 3, 1–24. [Google Scholar] [CrossRef]
- Sattelmacher, B. The apoplast and its significance for plant mineral nutrition. New Phytol. 2001, 149, 167–192. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Opatrilova, R.; Zehnalek, J.; Havel, L.; Kizek, R. Uncommon heavy metals, metalloids and their plant toxicity: A review. Environ. Chem. Lett. 2008, 6, 189–213. [Google Scholar] [CrossRef]
- Cass, H. Herbs for the nervous system: Ginko, kava, valerian, passionflower. Semin. Integr. Med. 2004, 2, 82–88. [Google Scholar] [CrossRef]
- Jambor, J. Growing herbs and herbal processing in Poland—Current state and development perspectives. Herba Pol. 2007, 53, 22–24. [Google Scholar]
- Miraj, S.; Rafieian, K.; Kiani, S. Melissa officinalis L.: A review study with an antioxidant prospective. J. Evid. Based Complement. Alter Med. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchia, K.; Dormana, H.J.D.; Oinonena, P.P.; Darwisd, Y.; Laakso, I.; Hiltunen, R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT 2008, 41, 391–400. [Google Scholar] [CrossRef]
- Mihajlov, L.; Ilieva, V.; Markova, N.; Zlatkovski, V. Organic cultivation of lemon ballm (Melissa officinalis) in Macedonia. J. Agric. Sci. Technol. B 2013, 3, 769–775. [Google Scholar]
- Dastmalchi, K.; Ollilainen, V.; Lackman, P.; Boije af Gennas, G.; Dorman, D.H.J.; Jarvinen, P.P.; Yli-Kauhaluoma, J.; Hiltunen, R. Acetylcholinesterase inhibitory guided fractionation of Melissa officinalis L. Bioorg. Med. Chem. 2009, 17, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Melissa officinalis and rosmarinic acid in management of memory functions and Alzheimer disease. Asian Pac. J. Trop. Biomed. 2019, 9, 47–52. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharm. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.J. Effects of cultivation systems on the growth, and essential oil content and composition of valerian. J. Herbs Spices Med. Plants 2008, 14, 54–67. [Google Scholar] [CrossRef]
- Peirce, A. The American Pharmaceutical Association Practical Guide to Natural Medicines; William Morrow and Company: New York, NY, USA, 1999. [Google Scholar]
- WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2007.
- Alloway, B.J. The General Monograph Herbal Drugs (1433). Pharmeuropa 2008, 20, 302–303. [Google Scholar]
- Miotto, A.; Ceretta, C.A.; Brunetto, G.; Nicoloso, F.T.; Girotto, E.; Farias, J.G.; Tiecher, T.L.; De Conti, L.; Trentin, G. Copper uptake, accumulation and physiological changes in adult grapevines in response to excess copper in soil. Plant. Soil 2014, 374, 593–610. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Lisowska, K.; Romanowska-Duda, Z.; Wolf, W.M. Combined cadmium-zinc interactions alter manganese, lead, copper uptake by Melissa officinalis. Scientific Reports, under review.
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar]
- Directive, C. Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Offic. J. Eur. Comm. 1986, 118, 0006–0012. [Google Scholar]
- IUSS Working Group. WRB World Reference Base for Soil Resources (2006); World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, L.; Du, Z.; Sun, X.; Amombo, E.; Fan, J.; Fu, J. Effects of cadmium exposure on growth and metabolic profile of Bermudagrass (Cynodon dactylon L. Pers.). PLoS ONE 2014, 29, e115279. [Google Scholar] [CrossRef]
- Piršelová, B.; Kuna, R.; Lukáč, P.; Havrlentová, M. Effect of cadmium on growth, photosynthetic pigments, iron and cadmium accumulation of faba bean (Vicia Faba cv. Aštar). Agriculture (Polnohospodárstvo) 2016, 62, 72–79. [Google Scholar] [CrossRef]
- Shah, F.R.; Ahmad, N.; Masood, K.R.; Zahid, D.M. The influence of cadmium and chromium on the biomass production of shisham (Dalbergia Sissoo Roxb.) seedlings. Pak. J. Bot. 2008, 40, 1341–1348. [Google Scholar]
- Ibrahim, M.H.; Kong, Y.C.; Zain, N.A.M. Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant Sambung Nyawa (Gynura procumbens (Lour.) Merr.). Molecules 2017, 22, 1623. [Google Scholar] [CrossRef]
- Feng, J.; Lin, Y.; Yang, Y.; Shena, Q.; Huanga, J.; Wang, S.; Zhu, X.; Li, Z. Tolerance and bioaccumulation of Cd and Cu in Sesuvium portulacastrum. Ecotoxicol. Environ. Saf. 2018, 147, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Balestri, M.; Ceccarini, A.; Forino, L.M.C.; Zelko, I.; Martinka, M.; Lux, A.; Castiglione, M.R. Cadmium uptake, localization and stress-induced morphogenic response in the fern Pteris vittata. Planta 2014, 239, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- De Maria, S.; Puschenreiter, M.; Rivelli, A.R. Cadmium accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. Plant. Soil Environ. 2013, 59, 254–261. [Google Scholar] [CrossRef]
- Burzyński, M.; Migocka, M.; Kłobus, G. Cu and Cd transport in cucumber (Cucumis sativus L.) root plasma membranes. Plant. Sci. 2005, 168, 1609–1614. [Google Scholar] [CrossRef]
- Žaltauskaite, J.; Šliumpaite, I. Evaluation of toxic effects and bioaccumulation of cadmium and copper in Spring Barley (Hordeum vulgare L. ) Environ. Res. Eng. Manag. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, X.; Li, T.; Hu, S.; Ji, J.; Wang, C. Characteristics of heavy metal transfer and their influencing factor in different soil-crop systems of the industrialization region, China. Ecotoxicol. Environ. Saf. 2016, 126, 193–201. [Google Scholar] [CrossRef]
- Galal, T.M.; Shehata, H.S. Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol. Indic. 2015, 48, 244–251. [Google Scholar] [CrossRef]
- Liu, K.; Lv, J.; He, W.; Zhang, H.; Cao, Y.; Dai, Y. Major factors influencing cadmium uptake from the soil into wheat plants. Ecotoxicol. Environ. Saf. 2015, 113, 207–213. [Google Scholar] [CrossRef]
- Skiba, E.; Kobyłecka, J.; Wolf, W.M. Influence of 2,4-D and MCPA herbicides on uptake and translocation of heavy metals in wheat (Triticum aestivum L.). Environ. Poll. 2017, 220, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Testiati, E.; Parinet, J.; Massiani, C.; Laffont-Schwob, I.; Rabier, J.; Pfeifer, H.-R.; Lenoble, V.; Masotti, V.; Prudent, P. Trace metal and metalloid contamination levels in soils and two native plant species of a former industrial site: Evaluation of the phytostabilization potential. J. Hazard. Mater. 2013, 248–249, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Bai, J.; Lu, Q.; Zhao, Q.; Gao, Z.; Wen, X.; Liu, X. Fractionation, transfer and ecological risks of heavy metals in riparian and ditch wetlands across a 100-year chronsequence of reclamation in estuary of China. Sci. Total Environ. 2015, 517, 66–75. [Google Scholar] [CrossRef]
- PN-ISO 10381-4:2007. Soil Quality—Sampling—Part 4: Rules for Procedure During the Research Areas of Natural, Semi-Natural and Cultivated. 2007. Available online: http://sklep.pkn.pl/pn-iso-10381-4-2007p.html (accessed on 15 May 2019).
- PN-ISO 10390:1997. Agricultural Chemical Analysis of the Soil. Determination of pH. 1997. Available online: http://sklep.pkn.pl/pn-iso-10390-1997p.html (accessed on 20 April 2019).
- ASTM D2974-00. Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils. Method D 2974–00; American Society for Testing and Materials: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; United States Environmental Protection Agency Environmental Sciences Division National Exposure Research Laboratory: Las Vegas, NV, USA, 2002.
- PN-ISO 11259:2001. Soil Quality—A Simplified Description of the Soil. 2001. Available online: http://sklep.pkn.pl/pn-iso-11259-2001p.html (accessed on 5 May 2019).
- Adamczyk-Szabela, D.; Markiewicz, J.; Wolf, W.M. Heavy metal uptake by herbs. IV. Influence of soil pH on the content of heavy metals in Valeriana officinalis L. Water Air Soil Pollut. 2015, 226, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Dybczyński, R.; Danko, B.; Kulisa, K.; Maleszewska, E.; Polkowska- Motrenko, H.; Samczyński, Z.; Szopa, Z. Preparation and preliminary certification of two new Polish CRMs for inorganic trace analysis. J. Radioanal. Nucl. Chem. 2004, 259, 409–413. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-Duda, Z.B. Ability of Cyanobacteria and microalgae in improvement of metabolic activity and development of willow plants. Pol. J. Environ. Stud. 2015, 24, 1003–1006. [Google Scholar] [CrossRef]
- Piotrowski, K.; Romanowska-Duda, Z.B.; Grzesik, M. How Biojodis and cyanobacteria alleviate the negative influence of predicted environmental constraints on growth and physiological activity of corn plants. Pol. J. Environ. Stud. 2016, 25, 741–751. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as an early indicator of light stress in barley. J. Photochem. Photobiol. B 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2016, 122, 121–127. [Google Scholar] [CrossRef]
- Goodson, D.Z. Mathematical Methods for Physical and Analytical Chemistry; Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro–Wilk, Kolmogorov–Smirnov, Lilliefors and Anderson–Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
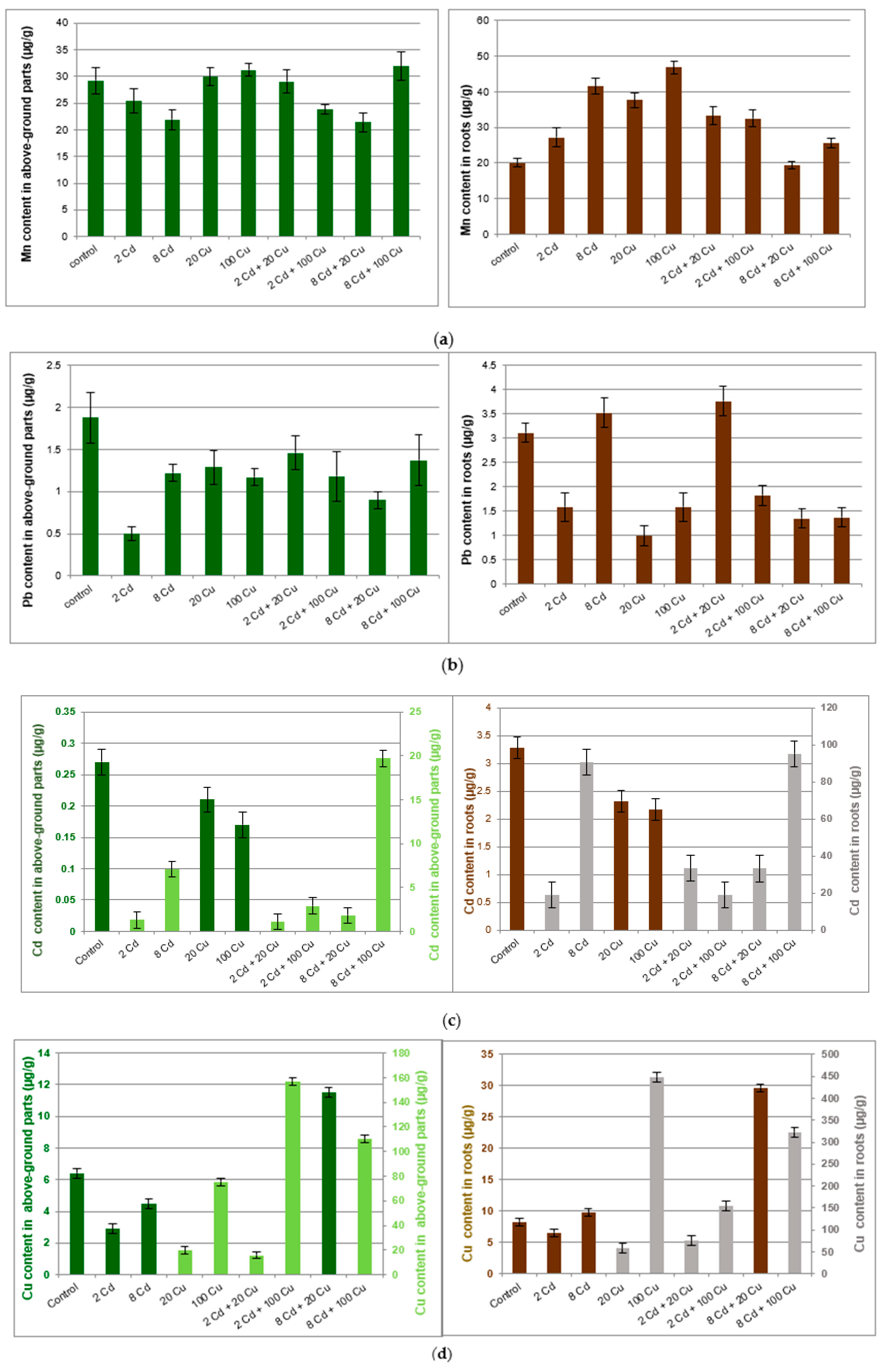
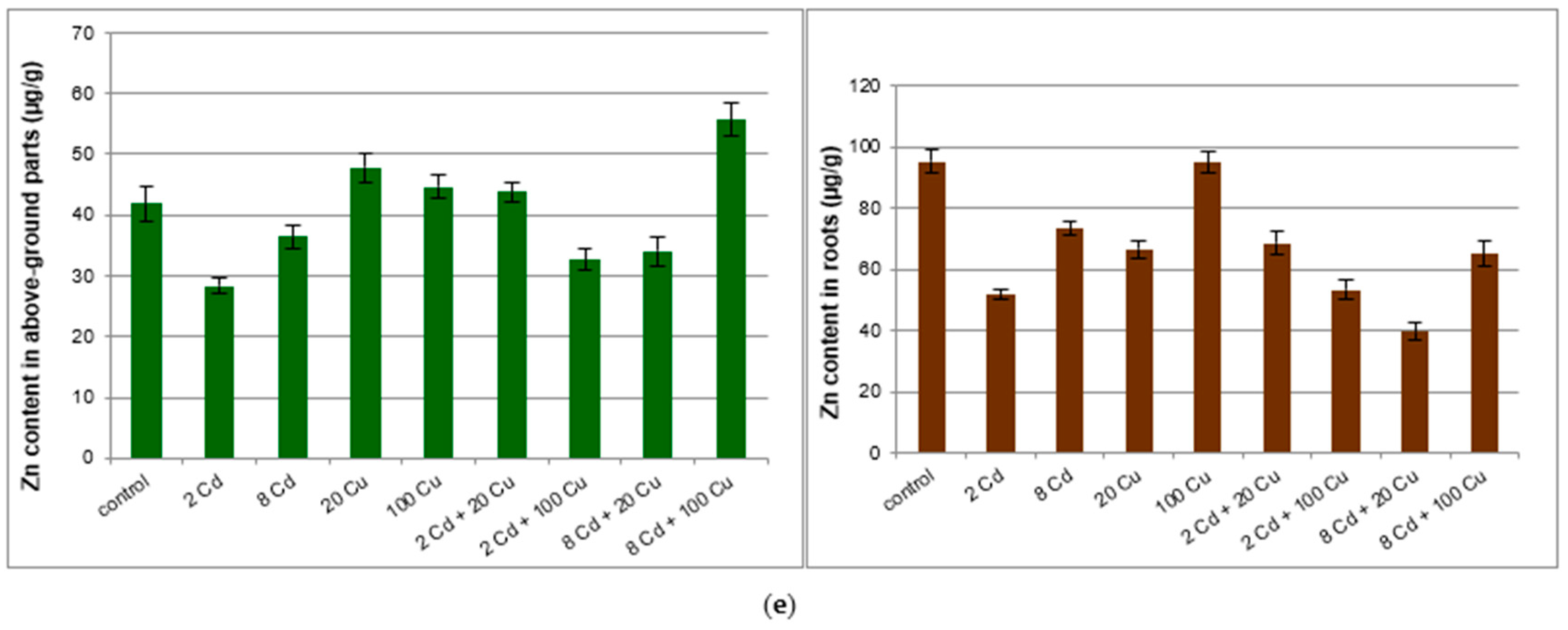

| Roots | ||||||
| Source of variation | SS | df | MS | F | p | Test F |
| Samples | 7961.42 | 8 | 995.18 | 207.72 | 6.35 × 10−62 | 2.0252 |
| Metals | 96,997.42 | 2 | 48,498.71 | 10,123.28 | 1.40 × 10−123 | 3.0803 |
| Interactions | 9687.58 | 16 | 605.47 | 126.38 | 3.59 × 10−62 | 1.7380 |
| Above ground parts | ||||||
| Source of variation | SS | df | MS | F | p | Test F |
| Samples | 2514.20 | 8 | 314.28 | 106.00 | 1.49 × 10−47 | 2.0252 |
| Metals | 36,578.72 | 2 | 18,289.36 | 6168.95 | 4.70 × 10−112 | 3.0803 |
| Interactions | 2080.99 | 16 | 130.06 | 43.87 | 9.17 × 10−40 | 1.7380 |
| Metal | Certified Value µg g−1 | Found µg g−1 | Recovery % |
|---|---|---|---|
| Manganese | 191 ± 12 | 188 ± 8 | 98 |
| Lead | 2.16 ± 0.23 | 2.13 ± 0.13 | 98 |
| Copper | 7.77 ± 0.53 | 7.50 ± 0.38 | 96 |
| Cadmium | 0.199 ± 0.015 | 0.206 ± 0.007 | 103 |
| Zinc | 33.5 ± 2.1 | 34.2 ± 0.7 | 102 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk-Szabela, D.; Lisowska, K.; Romanowska-Duda, Z.; Wolf, W.M. Associated Effects of Cadmium and Copper Alter the Heavy Metals Uptake by Melissa Officinalis. Molecules 2019, 24, 2458. https://doi.org/10.3390/molecules24132458
Adamczyk-Szabela D, Lisowska K, Romanowska-Duda Z, Wolf WM. Associated Effects of Cadmium and Copper Alter the Heavy Metals Uptake by Melissa Officinalis. Molecules. 2019; 24(13):2458. https://doi.org/10.3390/molecules24132458
Chicago/Turabian StyleAdamczyk-Szabela, Dorota, Katarzyna Lisowska, Zdzisława Romanowska-Duda, and Wojciech M. Wolf. 2019. "Associated Effects of Cadmium and Copper Alter the Heavy Metals Uptake by Melissa Officinalis" Molecules 24, no. 13: 2458. https://doi.org/10.3390/molecules24132458






