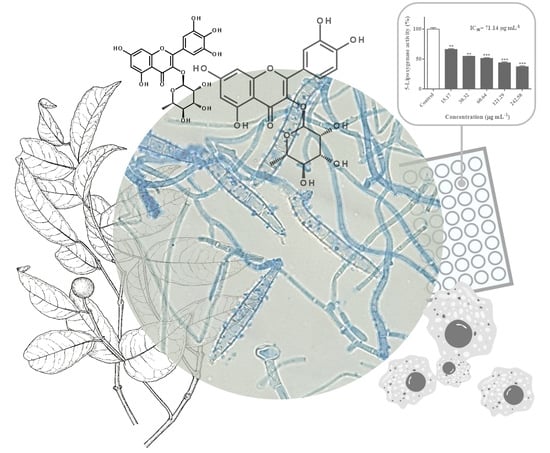
Flavonoid Composition of Salacia senegalensis (Lam.) DC. Leaves, Evaluation of Antidermatophytic Effects, and Potential Amelioration of the Associated Inflammatory Response
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Flavonoid Profile of S. senegalensis Leaves
2.2. Antimicrobial Effects
2.3. Inhibitory Effects Against 5-Lipoxygenase Activity
2.4. Effects Against RAW 264.7 Cells’ Viability and NO Levels
2.5. Effects Against HaCaT Cells’ Viability
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. HPLC-DAD Characterization of S. senegalensis Leaf Hydroethanolic Extract
3.3. Antimicrobial Activity Evaluation
3.3.1. Microbial Strains and Media
3.3.2. Antimicrobial Susceptibility Testing by Broth Microdilution
Antibacterial Susceptibility Testing
Antifungal Susceptibility Testing
3.4. Inhibition of 5-Lipoxygenase
3.5. Interference with RAW 264.7 Macrophages
3.5.1. RAW 264.7 Cells Culture and Viability
3.5.2. Determination of NO Levels
3.6. HaCaT Cells Culture and Viability
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferreres, F.; Gomes, N.G.; Valentao, P.; Pereira, D.M.; Gil-Izquierdo, A.; Araújo, L.; Silva, T.C.; Andrade, P.B. Leaves and stem bark from Allophylus africanus P. Beauv.: An approach to anti-inflammatory properties and characterization of their flavonoid profile. Food Chem. Toxicol. 2018, 118, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Mouho, D.G.; Oliveira, A.P.; Kodjo, C.G.; Valentão, P.; Ouattara, Z.A.; Bekro, Y.-A.; Andrade, P.B. Valorisation of Mangifera indica crop biomass residues. Ind. Crop. Prod. 2018, 124, 284–293. [Google Scholar] [CrossRef]
- Mouho, D.G.; Oliveira, A.P.; Kodjo, C.G.; Valentão, P.; Gil-Izquierdo, A.; Andrade, P.B.; Ouattara, Z.A.; Bekro, Y.-A.; Ferreres, F. Chemical findings and in vitro biological studies to uphold the use of Ficus exasperata Vahl leaf and stem bark. Food Chem. Toxicol. 2018, 112, 134–144. [Google Scholar] [CrossRef]
- Catarino, L.; Havik, P.J.; Romeiras, M.M. Medicinal plants of Guinea-Bissau: Therapeutic applications, ethnic diversity and knowledge transfer. J. Ethnopharmacol. 2016, 183, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Adumanya, O.C.U.; Uwakwe, A.A.; Essien, E.B. Essential oil composition (terpenes) of Salacia senegalensis Lam (DC) leaf. Br. J. Res. 2014, 1, 26–34. [Google Scholar]
- Adumanya, O.C.U.; Obiloma, A.A.; Essien, E.B. Proximate analysis, vitamins and mineral composition of Salacia senegalensis Lam (DC) leaves. Open J. Res. 2015, 2, 92–105. [Google Scholar]
- Balde, M.A.; Traore, M.S.; Diane, S.; Diallo, M.S.T.; Tounkara, T.M.; Camara, A.; Baldé, E.S.; Bah, F.; Ouedraogo, U.; Drame, H.; et al. Ethnobotanical survey of medicinal plants traditionally used in Low and Middle-Guinea for the treatment of skin diseases. J. Plant Sci. 2015, 3, 32–39. [Google Scholar]
- Svetaz, L.; Zuljan, F.; Derita, M.; Petenatti, E.; Tamayo, G.; Cáceres, A.; Filho, V.C.; Giménez, A.; Pinzón, R.; Zacchino, S.A.; et al. Value of ethnomedical information for the discovery of plants with antifungal properties. A survey among seven Latin American countries. J. Ethnopharmacol. 2010, 127, 137–158. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Peres, N.T.; Rossi, A. Pathogenesis of dermatophytosis: Sensing the host tissue. Mycopathologia 2017, 182, 215–227. [Google Scholar] [CrossRef]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef]
- Vázquez-Torres, A.; Balish, E. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 1997, 61, 170–192. [Google Scholar] [PubMed]
- Hube, B.; Hay, R.; Brasch, J.; Veraldi, S.; Schaller, M. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J. Mycol. Méd. 2015, 25, e44–e58. [Google Scholar] [CrossRef] [PubMed]
- Bagnazari, M.; Saidi, M.; Chandregowda, M.M.; Prakash, H.S.; Nagaraja, G. Phyto-constituents, pharmacological properties and biotechnological approaches for conservation of the anti-diabetic functional food medicinal plant Salacia: A review note. Appl. Food Biotechnol. 2017, 4, 1–10. [Google Scholar]
- Bagri, P.; Chester, K.; Khan, W.; Ahmad, S. Aspects of extraction and biological evaluation of naturally occurring sugar-mimicking sulfonium-ion and their synthetic analogues as potent α-glucosidase inhibitors from Salacia: A review. RSC Adv. 2017, 7, 28152–28185. [Google Scholar] [CrossRef]
- Jayawardena, M.; De Alwis, N.; Hettigoda, V.; Fernando, D. A double blind randomised placebo controlled cross over study of a herbal preparation containing Salacia reticulata in the treatment of type 2 diabetes. J. Ethnopharmacol. 2005, 97, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Adumanya, O.C.U. Carotenoids, phenolics, hydroxycinnamic acids and tannin composition of Salacia senegalensis (Lam) DC leaves. Nat. Prod. Chem. Res. 2016, 5, 1. [Google Scholar]
- Markham, K.R.; Bloor, S.J. Analysis and identification of flavonoids. In The Flavonoids - Advances in Research, 1st ed.; Harborne, J.B., Mabry, T.J., Eds.; Chapman and Hall: London, UK, 1998; pp. 19–134. [Google Scholar]
- Essien, A.D.; Akuodor, G.C.; Ajoku, G.A.; Megwas, A.U.; Anele, D.O.; Ezeunala, M.N.; Okezie, A.O. Antimicrobial and toxicological evaluation of ethanol leaf extract of Salacia lehmbachii. Interdiscip. Toxicol. 2017, 10, 163–167. [Google Scholar] [CrossRef]
- Kannaiyan, M.; Manuel, V.N.; Raja, V.; Thambidurai, P.; Mickymaray, S.; Nooruddin, T. Antimicrobial activity of the ethanolic and aqueous extracts of Salacia chinensis Linn. against human pathogens. Asian Pac. J. Trop. Dis. 2012, 2, S416–S420. [Google Scholar] [CrossRef]
- Subhasree, N.; Shivakumar, S.; Sandhiya, A.A.; Dubey, G.P. Vitro assessment of antioxidant and antibacterial activities of Salacia species - A comparative study. Int. J. Pharm. Pharm. Sci. 2013, 5, 279–282. [Google Scholar]
- Rodrigues, V.G.; Duarte, L.P.; Silva, R.R.; Silva, G.D.F.; Mercadante-Simões, M.O.; Takahashi, J.A.; Matildes, B.L.G.; Fonseca, T.H.S.; Gomes, M.A.; Filho, S.A.V. Salacia crassifolia (Celastraceae): CHEMICAL CONSTITUENTS AND ANTIMICROBIAL ACTIVITY. Química Nova 2015, 38, 237–242. [Google Scholar] [CrossRef]
- Gupta, A.K.; Foley, K.A.; Versteeg, S.G. New antifungal agents and new formulations against dermatophytes. Mycopathologia 2017, 182, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Pinto, E.; Salgueiro, L. Natural products: An alternative to conventional therapy for dermatophytosis? Mycopathologia 2017, 182, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tracanna, M.I.; Fortuna, A.M.; Cardenas, A.V.C.; Marr, A.K.; McMaster, W.R.; Gómez-Velasco, A.; Sánchez-Arreola, E.; Hernández, L.R.; Bach, H. Anti-leishmanial, anti-inflammatory and antimicrobial activities of phenolic derivatives from Tibouchina paratropica. Phytother. Res. 2015, 29, 393–397. [Google Scholar] [CrossRef]
- Ghani, S.B.A.; Weaver, L.; Zidan, Z.H.; Ali, H.M.; Keevil, C.W.; Brown, R.C. Microwave-assisted synthesis ant antimicrobial activities of flavonoid derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 518–522. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Andoh, T.; Haza, S.; Saito, A.; Kuraishi, Y. Involvement of leukotriene B4 in spontaneous itch-related behaviour in NC mice with atopic dermatitis-like skin lesions. Exp. Dermatol. 2011, 20, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Krieg, P.; Fürstenberger, G. The role of lipoxygenases in epidermis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2014, 1841, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Nicol, K.; Batra, R. Role of Antifungal Agents in the Treatment of Seborrheic Dermatitis. Am. J. Clin. Dermatol. 2004, 5, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.F.; Diehl, R.E.; Opas, E.; Rands, E.; Vickers, P.J.; Evans, J.F.; Gillard, J.W.; Miller, D.K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature 1990, 343, 282–284. [Google Scholar] [CrossRef]
- Percival, M.D. Human 5-lipoxygenase contains an essential iron. J. Biol. Chem. 1991, 266, 10058–10061. [Google Scholar] [PubMed]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.; Porto, G.; Cabrita, E.J.; Marques, M.M.B.; Fernandes, E. Inhibition of LOX by flavonoids: a structure–activity relationship study. Eur. J. Med. Chem. 2014, 72, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, N.; Pinto, A.; Capasso, F. Flavonoids, leucocyte migration and eicosanoids. J. Pharm. Pharmacol. 1998, 40, 293–295. [Google Scholar] [CrossRef]
- Winekenstädde, D.; Angelis, A.; Waltenberger, B.; Schwaiger, S.; Tchoumtchoua, J.; König, S.; Werz, O.; Aligiannis, N.; Skaltsounis, A.-L.; Stuppner, H. Phytochemical Profile of the Aerial Parts of Sedum sediforme and Anti-inflammatory Activity of Myricitrin. Nat. Prod. Commun. 2015, 10, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, Y.C.; Chung, S.K. Identification and in vitro biological activities of flavonols in garlic leaf and shoot: Inhibition of soybean lipoxygenase and hyaluronidase activities and scavenging of free radicals. J. Sci. Food Agric. 2005, 85, 633–640. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.; Mira, L.; Corvo, M.L. Molecular Mechanisms of Anti-Inflammatory Activity Mediated by Flavonoids. Curr. Med. Chem. 2008, 15, 1586–1605. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: structure–activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Noverr, M.C.; Toews, G.B.; Huffnagle, G.B. Production of Prostaglandins and Leukotrienes by Pathogenic Fungi. Infect. Immun. 2002, 70, 400–402. [Google Scholar] [CrossRef] [Green Version]
- Werz, O. Inhibition of 5-Lipoxygenase Product Synthesis by Natural Compounds of Plant Origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef] [Green Version]
- Baltazar, L.M.; Krausz, A.E.; Souza, A.C.O.; Adler, B.L.; Landriscina, A.; Musaev, T.; Nosanchuk, J.D.; Friedman, A.J. Trichophyton rubrum is Inhibited by Free and Nanoparticle Encapsulated Curcumin by Induction of Nitrosative Stress after Photodynamic Activation. PLoS ONE 2015, 10, e0120179. [Google Scholar] [CrossRef]
- Barker, J.; Griffiths, C.; Mitra, R.; Dixit, V.; Nickoloff, B.; Griffiths, C. Keratinocytes as initiators of inflammation. Lancet 1991, 337, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Hesselink, J.M.K.; Kopsky, D.J.; Bhaskar, A.K. Skin matters! The role of keratinocytes in nociception: A rational argument for the development of topical analgesics. J. Pain. Res. 2017, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hipler, U.-C. Evaluation of Biocompatibility and Cytotoxicity Using Keratinocyte and Fibroblast Cultures. Ski. Pharmacol. Physiol. 2009, 22, 74–82. [Google Scholar] [CrossRef] [PubMed]
- John, H.R.; Barbara, D.A.; David, A. Reference Method For Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- John, H.R.; Barbara, D.A.; David, A. Reference Methods For Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Jean, B.P.; Franklin, R.C.; Patricia, A.B. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Kachmar, M.R.; Oliveira, A.P.; Valentão, P.; Gil-Izquierdo, A.; Domínguez-Perles, R.; Ouahbi, A.; Badaoui, K.E.; Andrade, P.B.; Ferreres, F. HPLC-DAD-ESI/MSn phenolic profile and in vitro biological potential of Centarium erythraea Rafn aqueous extract. Food Chem. 2019, 278, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Duangsrisai, S.; Gomes, N.G.; Suksungworn, R.; Pereira, D.M.; Gil-Izquierdo, A.; Valentão, P.; Choowongkomon, K.; Andrade, P.B. Anti-inflammatory properties of the stem bark from the herbal drug Vitex peduncularis Wall. ex Schauer and characterization of its polyphenolic profile. Food Chem. Toxicol. 2017, 106, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.M.; Fernandes, F.; Madureira-Carvalho, Á.; Valentão, P.; Lobo-Da-Cunha, A.; Calado, G.; Andrade, P.B. Profiling of Heterobranchia Sea Slugs from Portuguese Coastal Waters as Producers of Anti-Cancer and Anti-Inflammatory Agents. Molecules 2018, 23, 1027. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 3, 6 and 7 are available from the authors. |




| Peak | Compounds | UV (nm) | mg Kg−1 Dry Extract |
|---|---|---|---|
| 1 | Myricitrin | 259, 298sh, 351 | 10165.70 ± 119.98 |
| 2 | Flavonoid derivative | 257, 266sh, 355 | 916.35 ± 85.12 |
| 3 | Isoquercitrin | 257, 266sh, 353 | 1096.10 ± 48.29 |
| 4 | Flavonoid derivative | 257, 267sh, 355 | 731.05 ± 38.75 |
| 5 | Flavonoid derivative | 256, 266sh, 294sh, 354 | 2741.27 ± 113.18 |
| 6 | Quercetin-3-O-xyloside | 256, 265sh, 294sh, 354 | 9424.36 ± 213.32 |
| 7 | Quercitrin | 260, 306sh, 352 | 54675.24 ± 1352.64 |
| Total | 79750.07 ± 1971.28 | ||
| Microorganism | MIC a | MLC b |
|---|---|---|
| Fungi | ||
| Candida albicans ATCC® 10231 | >4 | >4 |
| Candida albicans D5 | >4 | >4 |
| Malassezia furfur P26 | 4 | >4 |
| Aspergillus fumigatus ATCC® 204305 | >4 | >4 |
| Scopulariopsis brevicaulis FF | >4 | >4 |
| Epidermophyton floccosum FF9 | 1 | 4 |
| Microsporum canis FF1 | 2 | 4 |
| Microsporum gypseum FF3 | 4 | >4 |
| Trichophyton mentagrophytes FF7 | 2 | 4 |
| Trichophyton rubrum FF5 | 1 | 2 |
| Bacteria | ||
| Escherichia coli ATCC® 25922 | >4 | >4 |
| Staphylococcus aureus ATCC® 25923 | 2 | >4 |
| Standard | Regression Equation | Linearity Range (µg mL−1) | LOD (µg mL−1) | LOQ (µg mL−1) | ||
|---|---|---|---|---|---|---|
| Slope (S) | Intercept (b) | R2 (n=3) | ||||
| Myricitrin | 44.893 | 831.50 | 0.998 | 25–400 | 4.78 | 14.49 |
| Isoquercitrin | 59.725 | 694.77 | 0.996 | 12.5–200 | 0.507 | 1.537 |
| Quercitrin | 48.9 | 1085.9 | 0.997 | 25–400 | 0.591 | 1.792 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, N.G.M.; Oliveira, A.P.; Cunha, D.; Pereira, D.M.; Valentão, P.; Pinto, E.; Araújo, L.; Andrade, P.B. Flavonoid Composition of Salacia senegalensis (Lam.) DC. Leaves, Evaluation of Antidermatophytic Effects, and Potential Amelioration of the Associated Inflammatory Response. Molecules 2019, 24, 2530. https://doi.org/10.3390/molecules24142530
Gomes NGM, Oliveira AP, Cunha D, Pereira DM, Valentão P, Pinto E, Araújo L, Andrade PB. Flavonoid Composition of Salacia senegalensis (Lam.) DC. Leaves, Evaluation of Antidermatophytic Effects, and Potential Amelioration of the Associated Inflammatory Response. Molecules. 2019; 24(14):2530. https://doi.org/10.3390/molecules24142530
Chicago/Turabian StyleGomes, Nelson G. M., Andreia P. Oliveira, Diana Cunha, David M. Pereira, Patrícia Valentão, Eugénia Pinto, Luísa Araújo, and Paula B. Andrade. 2019. "Flavonoid Composition of Salacia senegalensis (Lam.) DC. Leaves, Evaluation of Antidermatophytic Effects, and Potential Amelioration of the Associated Inflammatory Response" Molecules 24, no. 14: 2530. https://doi.org/10.3390/molecules24142530