The Chemistry and Biology of Cyclophostin, the Cyclipostins and Related Compounds
Abstract
:1. Introduction
2. Discussion
2.1. Isolation and Structure of Cyclophostin
2.2. Isolation of the Cyclipostins
2.3. Isolation of the Salinipostins
2.4. Stereochemical Nomenclature
2.5. Biosynthesis
2.6. Synthesis of (±) Cyclophostin and (±) Cyclipostin P
2.7. Synthesis of the Salinipostins
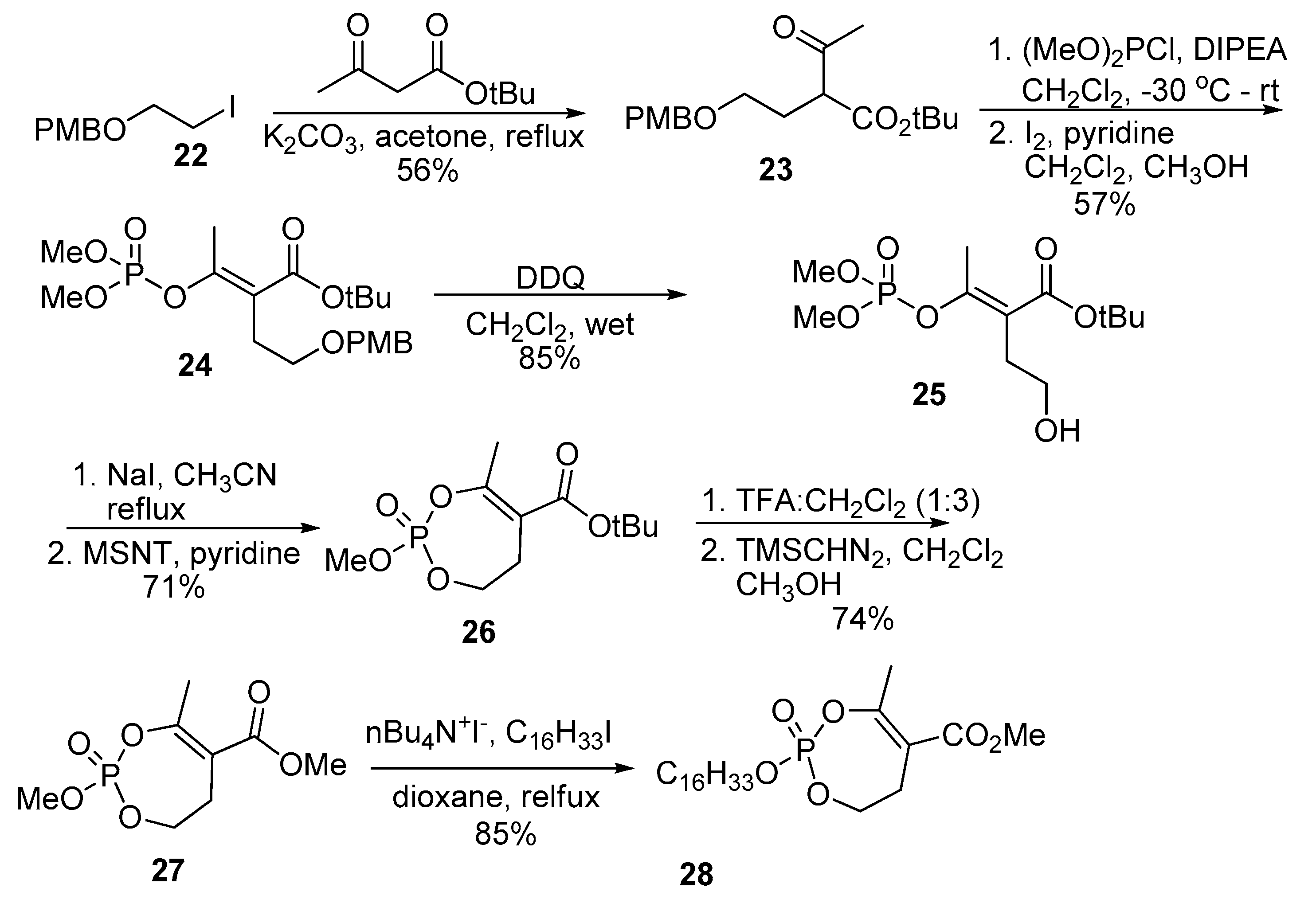
2.8. Synthesis of Mono and Bicyclic Phosphonate Analogs
2.9. Synthesis of Monocyclic Phosphate Analogs
2.10. Synthesis of α,α-difluoro Phosphonate Analogs
2.11. Biological Activities
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-emergence of natural products for drug discovery in the genomic era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.; Peter, H.H. Insecticidal organophosphates: Nature made them first. Experimentia 1987, 43, 1235–1237. [Google Scholar] [CrossRef]
- Kurokawa, T.; Suzuki, K.; Hayaoka, T.; Nakagawa, T.; Izawa, T.; Kobayashi, M.; Harada, N. Cyclophostin, Acetylcholinesterase inhibitor from Streptomyces lavendulae. J. Antibiot. 1993, 46, 1315–1318. [Google Scholar] [CrossRef] [PubMed]
- Wink, J.; Schmidt, F.-R.; Seibert, G.; Aretz, W. Cyclipostins: Novel hormone sensitive lipase inhibitors from Streptomyces sp. DSM 13381 I. Taxonomic studies of the producer microorganism and fermentation results. J. Antibiot. 2002, 55, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Vertesy, L.; Beck, B.; Brönstrup, M.; Ehrlich, K.; Kurz, M.; Müller, G.; Schummer, D.; Seibert, G. Cyclipostins: Novel hormone sensitive lipase inhibitors from Streptomyces sp. DSM 13381 II. Isolation, structure, elucidation and biological properties. J. Antibiot. 2002, 55, 480–494. [Google Scholar] [CrossRef]
- Vertesy, L.; Ehrlich, K.; Kurz, M.; Wink, J. Cyclopostins, Process for their Preparation and Use Thereof. U. S. Patent US 6,756,402 B2, 29 June 2004. [Google Scholar]
- Seibert, G.; Toti, L.; Wink, J. Treating Mycobacterial Infections with Cyclipostins. WO/2008/025449, 6 March 2008. [Google Scholar]
- Schulze, C.J.; Navarro, G.; Ebert, D.; DeRisi, J.; Linnington, R.G. Salinipostins A-K, long-chain bicyclic phosphotriesters as a potent and selective antimalarial chemotype. J. Org. Chem. 2015, 80, 1312–1320. [Google Scholar] [CrossRef]
- Malla, R.K.; Bandyopadhyay, S.; Spilling, C.D.; Dutta, S.; Dupureur, C.M. The first total synthesis of (±) cyclophostin and (±) cyclipostin P: Inhibitors of the serine hydrolases acetyl cholinesterase and hormone sensitive lipase. Org. Lett. 2011, 13, 3094–3097. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.K.; Prelog, V. Specification of molecular chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Morin, J.B.; Adams, K.L.; Sello, J.K. Replication of biosynthetic reactions enables efficient synthesis of A-Factor, a γ-butyrolactone autoinducer from Streptomyces griseus. Org. Biomol. Chem. 2012, 10, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Nihira, T.; Shimizu, Y.; Kim, H.S.; Yamada, Y. Structure-activity relationships of virginiae butanolide C, an inducer of virginiamycin production in Streptomyces virginiae. J. Antbiot. 1988, 1828–1837. [Google Scholar] [CrossRef]
- Sakuda, S.; Higashi, A.; Tanaka, S.; Nihira, T.; Yamada, Y. Biosynthesis of virginiae butanolide A, a butyrolactone autoregulator from Streptomyces. J. Am. Chem. Soc. 1992, 114, 663–668. [Google Scholar] [CrossRef]
- Sakuda, S.; Tanaka, S.; Mizuno, K.; Sukcharoen, O.; Nihira, T.; Yamada, Y. Biosynthetic studies on virginiae butanolide A, a butyrolactone autoregulator from Streptomyces. Part 2. Preparation of Possible Biosynthetic intermediates and conversion experiments in cell-free system. J. Chem. Soc. Perkin Trans. I 1993, 2309–2315. [Google Scholar] [CrossRef]
- Takano, E.; Nihira, T.; Hara, Y.; Jones, J.J.; Gershater, C.J.L.; Yamada, Y.; Bibb, M. Purification and structural determination of SCB1, a γ-butyrolactone that elicits antibiotic production in Streptomyces coelicolor A3(2). J. Biol. Chem. 2000, 275, 11010–11016. [Google Scholar] [CrossRef]
- Kato, J.; Funa, N.; Watanabe, H.; Ohnishi, Y.; Horinouchi, S. Biosynthesis of γ-butyrolactone autoregulators that switch on secondary metabolism and morphological development in Streptomyces. Proc. Natl. Acad. Sci. USA 2007, 104, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wei, X.; Liu, X.; Dong, X.; Yu, R.; Wan, S.; Jiang, T. Total synthesis of marine cyclic enol-phosphotriester salinipostin compounds. J. Ocean. Univ. China 2018, 17, 683–689. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Dutta, S.; Spilling, C.D.; Dupureur, C.M.; Rath, N.P. Synthesis and biological evaluation of a phosphonate analog of the natural acetyl cholinesterase inhibitor cyclophostin. J. Org. Chem. 2008, 73, 8386–8391. [Google Scholar] [CrossRef]
- Dutta, S.; Malla, R.K.; Bandyopadhyay, S.; Spilling, C.D.; Dupureur, C.M. Synthesis and kinetic analysis of some phosphonate analogs of cyclophostin as inhibitors of human acetylcholinesterase. Bioorg. Med. Chem. 2010, 18, 2265–2274. [Google Scholar] [CrossRef] [Green Version]
- Point, V.; Malla, R.K.; Diomande, S.; Martin, B.P.; Delorme, V.; Carrière, F.; Canaan, S.; Rath, N.P.; Spilling, C.D.; Cavalier, J.-F. Synthesis and kinetic evaluation of cyclophostin and cyclipostins phosphonate analogs as selective and potent inhibitors of microbial lipases. J. Med. Chem. 2012, 55, 10204–10219. [Google Scholar] [CrossRef]
- Point, V.; Malla, R.K.; Carrière, F.; Canaan, S.; Spilling, C.D.; Cavalier, J.-F. Enantioselective inhibition of microbial lipolytic enzymes by nonracemic monocyclic enolphosphonate analogs of cyclophostin. J. Med. Chem. 2013, 56, 4393–4401. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, E.; Dutta, S.; Malla, R.K.; Martin, B.P.; Spilling, C.D.; Dupureur, C.M. Rat hormone sensitive lipase inhibition by cyclipostins and their analogs. Bioorg. Med. Chem. 2015, 23, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.P.; Vasilieva, E.; Dupureur, C.M.; Spilling, C.D. Synthesis and comparison of the biological activity of monocyclic phosphonate, difluorophosphonate and phosphate analogs of the natural AChE inhibitor cyclophostin. Bioorg. Med. Chem. 2015, 23, 7529–7534. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.C.; Delorme, V.; Bénarouche, A.; Martin, B.P.; Paudel, R.; Gnawali, G.R.; Madani, A.; Puppo, R.; Landry, V.; Kremer, L.; et al. Cyclipostins and cyclophostin analogs as promising compounds in the fight against tuberculosis. Sci. Rep. 2017, 7, 11751. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.C.; Madani, A.; Santucci, P.; Martin, B.P.; Paudel, R.; Delattre, S.; Herrmann, J.-L.; Spilling, C.D.; Kremer, L.; Canaan, S. Cyclophostin and cyclipostins analogs, new promising molecules to treat mycobacterial-related diseases. Int. J. Antimicrob Agents 2018, 51, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Richard, M.; Nguyen, P.C.; Fourquet, P.; Camoin, L.; Paudal, R.; Gnawali, G.R.; Spilling, C.D.; Cavalier, J.-F.; Canaan, S.; et al. Cyclipostins and cyclophostin analogs inhibit the antigen 85C from Mycobacterium tuberculosis both in vitro and in vivo. J. Biol. Chem. 2018, 293, 2755–2769. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.C.; Nguyen, V.S.; Martin, B.P.; Fourquet, P.; Camoin, L.; Spilling, C.D.; Cavalier, J.-F.; Cambillau, C.; Canaan, S. Biochemical and structural characterization of TesA, a major thioesterase required for outer-envelope lipid biosynthesis in M. tuberculosis. J. Mol. Biol. 2018, 430, 5120–5136. [Google Scholar] [CrossRef]
- Santucci, P.; Point, V.; Poncin, I.; Guy, A.; Crauste, C.; Serveau-Avesque, C.; Galano, J.M.; Spilling, C.D.; Cavalier, J.F.; Canaan, S. LipG a bifunctional phospholipase/thioesterase involved in mycobacterial envelope remodeling. Biosci. Rep. 2018, 38, BSR20181953. [Google Scholar] [CrossRef]












© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spilling, C.D. The Chemistry and Biology of Cyclophostin, the Cyclipostins and Related Compounds. Molecules 2019, 24, 2579. https://doi.org/10.3390/molecules24142579
Spilling CD. The Chemistry and Biology of Cyclophostin, the Cyclipostins and Related Compounds. Molecules. 2019; 24(14):2579. https://doi.org/10.3390/molecules24142579
Chicago/Turabian StyleSpilling, Christopher D. 2019. "The Chemistry and Biology of Cyclophostin, the Cyclipostins and Related Compounds" Molecules 24, no. 14: 2579. https://doi.org/10.3390/molecules24142579




