Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine
Abstract
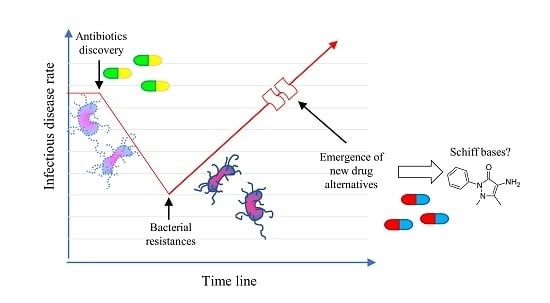
:1. Introduction
2. Results
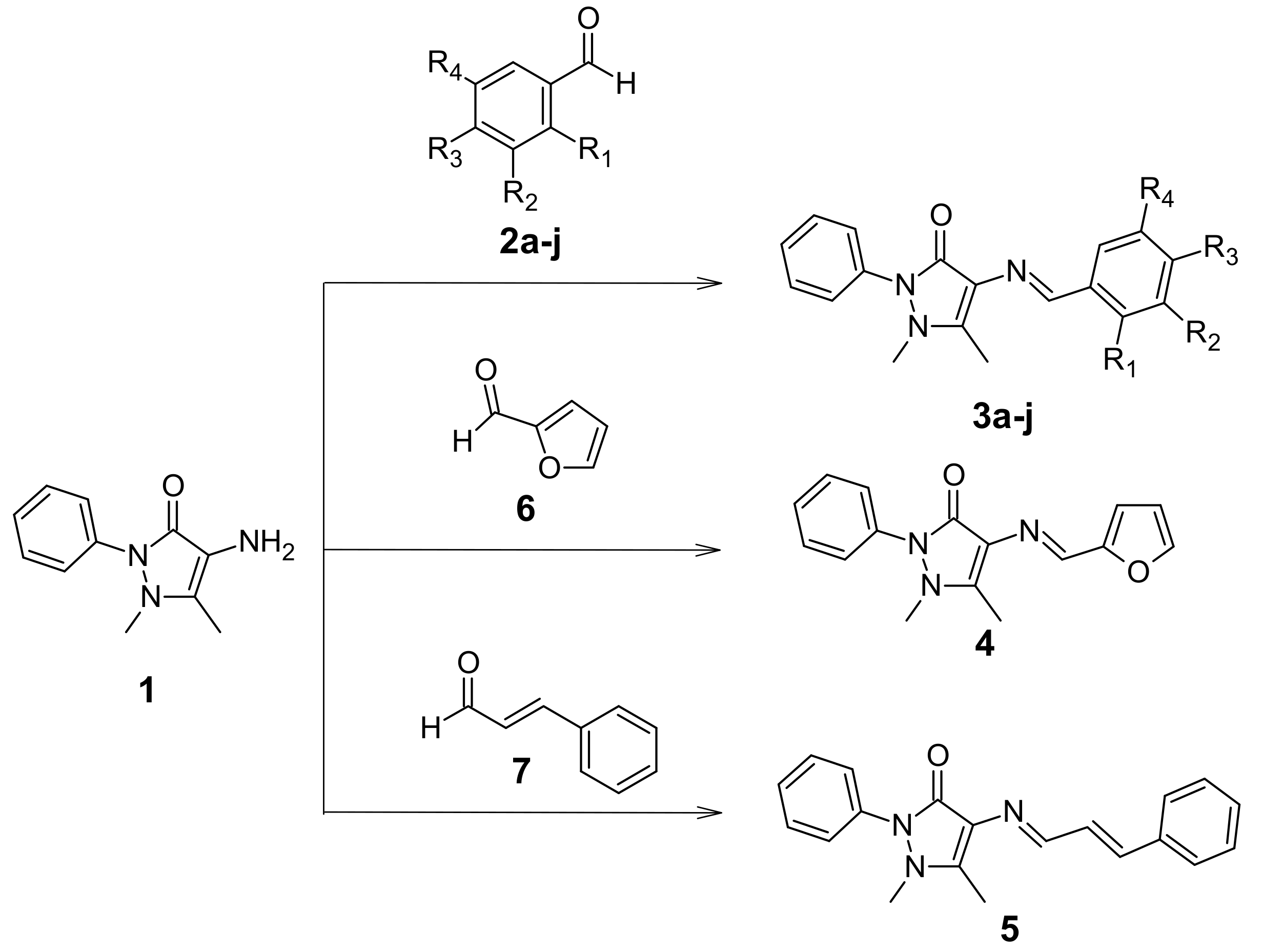
2.1. Synthesis of Schiff Base of 4-Aminoantipyrine
2.2. Antimicrobial Evaluation
2.3. Evaluation of Antioxidant/Oxidative Activity
2.4. Toxicity in Mammalian Cell Lines
2.5. Evaluation of Pharmacokinetic and Drug-Like Properties
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Synthesis of Schiff Base Derivatives (3a–j, 4, 5)
4.3. Evaluation of Antimicrobial Activity
4.4. Evaluation of Leishmanicidal Activity
4.5. Cell Viability
4.6. Drop Test
4.7. DPPH Radical Scavenging Assay
4.8. Theoretical Prediction of ADME Properties and Bioactivity Scores
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toutain, P.L.; Bousquet-Melou, A. The consequences of generic marketing on antibiotic consumption and the spread of microbial resistance: The need for new antibiotics. J. Vet. Pharmacol. Ther. 2013, 36, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Mehla, K.; Ramana, J. Structural signature of Ser83Leu and Asp87Asn mutations in DNA gyrase from enterotoxigenic Escherichia coli and impact on quinolone resistance. Gene 2016, 576, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, A.; Cantizani, J.; Peña, I.; Manzano, P.; Rijal, S.; Dujardin, J.C.; De Muylder, G.; Martin, J. Importance of secondary screening with clinical isolates for anti-leishmania drug discovery. Sci. Rep. 2018, 8, 11765. [Google Scholar] [CrossRef]
- Miller-Petrie, M.; Pant, S.; Laxminarayan, R. Drug-Resistante Infections. In Major Infectious Diseases, 3rd ed.; Holmes, K.K., Bertozzi, S., Bloom, B.R., Jha, P., Eds.; World Bank: Washington, DC, USA, 2017; Volume 6, pp. 433–448. [Google Scholar]
- Oldfield, E.; Feng, X. Resistance-Resistant Antibiotics. Trends Pharmacol. Sci. 2014, 35, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Selvaganapathy, M.; Sudharsan, S. DNA, the biopolymer as a target material for metalloinsertors: From chemistry to preclinical implications. Mater. Sci. Eng. C 2015, 53, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Al-Harrasi, A.S.; Hussain, H.; Hussain, J.; Rashid, R.; Choudhary, M.I. Antiglycation therapy: Discovery of promising antiglycation agents for the management of diabetic complications. Pharm. Biol. 2016, 54, 198–206. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Sharma, N.; Kamboj, S.; Saini, V. Therapeutic potential of hydrazones as anti-inflammatory agents. Int. J. Med. Chem. 2014, 2014, 761030. [Google Scholar] [CrossRef]
- Sonmez, F.; Gunesli, Z.; Kurt, B.Z.; Gazioglu, I.; Avci, D.; Kucukislamoglu, M. Synthesis, antioxidant activity and SAR study of novel spiro-isatin-based Schiff bases. Mol. Divers. 2019. [Google Scholar] [CrossRef]
- Zanon, V.S.; Lima, J.A.; Cuya, T.; Lima, F.R.S.; da Fonseca, A.C.C.; Gomez, J.G.; Ribeiro, R.R.; França, T.C.C.; Vargas, M.D. In-vitro evaluation studies of 7-chloro-4-aminoquinoline Schiff bases and their copper complexes as cholinesterase inhibitors. J. Inorg. Biochem. 2019, 191, 183–193. [Google Scholar] [CrossRef]
- Karrouchi, K.; Chemlal, L.; Taoufik, J.; Cherrah, Y.; Radi, S.; El Abbes Faouzi, M.; Ansar, M. Synthesis, antioxidant and analgesic activities of Schiff bases of 4-amino-1,2,4-triazole derivatives containing a pyrazole moiety. Ann. Pharm. Fr. 2016, 74, 431–438. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Saad, O.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M.; Matlob, A.A. Rational design and synthesis of new, high efficiency, multipotent Schiff base-1,2,4-triazole antioxidants bearing butylated hydroxytoluene moieties. Molecules 2016, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Choi, J.-H.; Lee, D.-U. Synthesis of novel Schiff base analogues of 4-amino-1, 5-dimethyl-2-phenylpyrazol-3-one and their evaluation for antioxidant and anti-inflammatory activity. Bioorg. Med. Chem. 2012, 20, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Anupama, B.; Sunita, M.; Shiva Leela, D.; Ushaiah, B.; Gyana Kumari, C. Synthesis, spectral characterization, DNA binding studies and antimicrobial activity of Co(II), Ni(II), Zn(II), Fe(III) and VO(IV) complexes with 4-aminoantipyrine schiff base of ortho-vanillin. J. Fluoresc. 2014, 24, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Ceruso, M.; Carta, F.; Osman, S.M.; Alothman, Z.; Monti, S.M.; Supuran, C.T. Inhibition studies of bacterial, fungal and protozoan β-class carbonic anhydrases with Schiff bases incorporating sulfonamide moieties. Bioorg. Med. Chem. 2015, 23, 4181–4187. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Khan, K.M. Schiff bases in medicinal chemistry: A patent review (2010–2015). Expert Opin. Ther. Pat. 2017, 27, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Munro, O.Q.; Akerman, K.J.; Akerman, P. Gold Complexes for use in the Treatment of Cancer. U.S. Patent 9,346,832, 24 May 2016. [Google Scholar]
- Raman, N.; Sakthivel, A.; Pravin, N. Exploring DNA binding and nucleolytic activity of few 4-aminoantipyrine based amino acid Schiff base complexes: A comparative approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 125, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Ali, S.; Raja, D. Designing, synthesis and spectral characterization of Schiff base transition metal complexes: DNA cleavage and antimicrobial activity studies. J. Serbian Chem. Soc. 2008, 73, 1063–1071. [Google Scholar] [CrossRef]
- Gabellieri, E.; Guba, W.; Hilpert, H.; Mauser, H.; Mayweg, A.V.; Rogers-Evans, M.; Rombach, D.; Thomas, A.; Woltering, T.; Wostl, W. 1,4-Oxazepines As Bace1 and/or Bace2 Inhibitors. U.S. Patent 8,748,418, 10 June 2014. [Google Scholar]
- Djaballah, H.; Wu, H.; Feldman, T.; Jiang, X. Allosteric reversible pan-caspase inhibitors. WO Patent 2012/134822 A1, 4 October 2012. [Google Scholar]
- Choudhary, M.I.; Khan, A.; Khan, K.M.; Ambreen, N.; Wahab, A.; Rahman, A. Schiff Bases of Thiazoles: A New Class of Ureases Inhibitors. U.S. Patent 9,447,057 B2, 20 September 2016. [Google Scholar]
- Ahmad, S.; Khan, M.S.; Akhter, F.; Ajid Khan, M.S.; Khan, A.; Ashraf, J.M.; Pandey, R.P.; Shahab, U. Glycoxidation of biological macromolecules: A critical approach to halt the menace of glycation. Glycobiology 2014, 24, 979–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldini, G.; Vistoli, G.; Stefek, M.; Chondrogianni, N.; Grune, T.; Sereikaite, J.; Sadowska-Bartosz, I.; Bartosz, G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013, 47, 93–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Resayes, S.I.; Warad, I.; Al-Nuri, M.A.; Choudhary, M.I.; Wahab, A.-T.; Rasheed, S. Heterocyclic schiff’s bases as novel and new antiglycation agents. U.S. Patent Application 13/757,956, 7 August 2014. [Google Scholar]
- Nagasawa, T.; Tabata, N.; Ito, Y.; Nishizawa, N.; Aiba, Y.; Kitts, D.D. Inhibition of glycation reaction in tissue protein incubations by water soluble rutin derivative. Mol. Cell. Biochem. 2003, 249, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Ueda, Y.; Asahi, K.; Izuhara, Y.; Inagi, R.; Saito, A.; Van Ypersele De Strihou, C.; Kurokawa, K. Mechanism of the inhibitory effect of OPB-9195 [(+/−)-2-isopropylidenehydrazono-4-oxo-thiazolidin-5-yla cetanilide] on advanced glycation end product and advanced lipoxidation end product formation. J. Am. Soc. Nephrol. 2000, 11, 1719–1725. [Google Scholar] [PubMed]
- Senbagam, R.; Vijayakumar, R.; Rajarajan, M.; Balaji, S.; Manikandan, V.; Vanangamudi, G.; Thirunarayanan, G. Synthesis, assessment of substituent effect and antimicrobial activities of (4E)-4-(benzylideneamino)-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one compounds. Karbala Int. J. Mod. Sci. 2016, 2, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.S.; Lee, D.-U.; Bari, L. Antibacterial and Cytotoxic Activities of Schiff Base Analogues of 4-Aminoantipyrine. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 613–619. [Google Scholar] [CrossRef]
- Sigroha, S.; Narasimhan, B.; Kumar, P.; Khatkar, A.; Ramasamy, K.; Mani, V.; Mishra, R.K.; Majeed, A.B.A. Design, synthesis, antimicrobial, anticancer evaluation, and QSAR studies of 4-(substituted benzylidene-amino)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-ones. Med. Chem. Res. 2012, 21, 3863–3875. [Google Scholar] [CrossRef]
- Ali, P.; Meshram, J.; Sheikh, J.; Tiwari, V.; Dongre, R.; Hadda, T.B. Predictions and correlations of structure activity relationship of some aminoantipyrine derivatives on the basis of theoretical and experimental ground. Med. Chem. Res. 2012, 21, 157–164. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Abd-Elwahab, Z.H.; Faheim, A.A. Preparation, Spectral Characterization and Antimicrobial Activities ofSchiff Base Complexes Derived from 4-Aminoantipyrine. Mixed LigandComplexes with 2-Aminopyridine, 8-Hydroxyquinoline and Oxalic Acidand their Pyrolytical Products. J. Chinese Chem. Soc. 2004, 51, 901–915. [Google Scholar] [CrossRef]
- Singh, O.P.; Sundar, S. Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: Current status and future prospects. Front. Immunol. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Zhang, R.; Ding, D.-J.; Liu, S.; Wang, B.-L.; Wang, Y.-L.; Lin, Y.-X. Experimental and density functional studies on two structurally similar antipyrine derivatives: 4-(2-hydroxy-5-nitrobenzylidene-amino)-1,2-dihydro-1,5-dimethyl-2-phenylpyrazol-3-one and 4-(3-bromo-5-chloro-2-hydroxybenzylideneamino)-1,2-dihydro-1,5-dimeth. Struct. Chem. 2006, 17, 655–665. [Google Scholar] [CrossRef]
- Bensaber, S.; Allafe, H.A.; Ermeli, N.; Mohamed, S.; Zetrini, A.; Alsabri, S.; Erhuma, M.; Hermann, A.; Jaeda, M.; Gbaj, A. Chemical synthesis, molecular modelling, and evaluation of anticancer activity of some pyrazol-3-one Schiff base derivatives. Med. Chem. Res. 2014, 23, 5120–5134. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tapia, E.; Nana, R.K.; Querol, A.; Pérez-Torrado, R. Ethanol cellular defense induce unfolded protein response in yeast. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Pączka, A.; Mołoń, M.; Bartosz, G. Dimethyl sulfoxide induces oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 13, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, E.; Iturrioz-Rodríguez, N.; Padín-González, E.; Villegas, J.; García-Hevia, L.; Pérez-Lorenzo, M.; Parak, W.J.; Correa-Duarte, M.A.; Fanarraga, M.L. Carbon nanotubes gathered onto silica particles lose their biomimetic properties with the cytoskeleton becoming biocompatible. Int. J. Nanomed. 2017, 12, 6317–6328. [Google Scholar] [CrossRef] [PubMed]
- Kalaydina, R.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727–4745. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Vinodkumar, C.R.; Radhakrishnan, P.K. Complexes of yttrium and lanthanide perchlorates with 4-N-(2´-furfurylidene)aminoantipyrine. Synth. React. Inorg. Met. Chem. 1997, 27, 1347–1355. [Google Scholar] [CrossRef]
- Edlind, T.; Smith, L.; Henry, K.; Katiyar, S.; Nickels, J. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signalling. Mol. Microbiol. 2002, 46, 257–268. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. In M07-A9 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, pp. 18–19. [Google Scholar]
- Nikzad, S.; Baradaran-Ghahfarokhi, M.; Nasri, P. Dose-response modeling using MTT assay: A short review. Life Sci. J. 2014, 11, 432–437. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Giroux-Leprieur, E.; Costantini, A.; Ding, V.; He, B. Hedgehog Signaling in Lung Cancer: From Oncogenesis to Cancer Treatment Resistance. Int. J. Mol. Sci. 2018, 19, 2835. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Ricci, G.; Severini, G.M.; Romano, F.; Biffi, S. Imaging and therapy of ovarian cancer: Clinical application of nanoparticles and future perspectives. Theranostics 2018, 8, 4279–4294. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.E.; Stratford, I.J.; Wallace, R.G.; Wardman, P.; Watts, M.E. Toxicity of Nitro Compounds Toward Hypoxic Mammalian Cells In Vitro: Dependence on Reduction Potential. J. Natl. Cancer Inst. 1980, 64, 555–560. [Google Scholar] [PubMed]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Wyllie, S.; Roberts, A.J.; Norval, S.; Patterson, S.; Foth, B.J.; Berriman, M.; Read, K.D.; Fairlamb, A.H. Activation of Bicyclic Nitro-drugs by a Novel Nitroreductase (NTR2) in Leishmania. PLoS Pathog. 2016, 12, e1005971. [Google Scholar] [CrossRef]
- Sobczyk, L.; Chudoba, D.; Tolstoy, P.M.; Filarowski, A. Some brief notes on theoretical and experimental investigations of intramolecular hydrogen bonding. Molecules 2016, 21, 1657. [Google Scholar] [CrossRef]
- Agudelo-Higuita, N.I.; Huycke, M.M. Enterococcal Disease, Epidemiology, and Implications for Treatment. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014; pp. 65–99. [Google Scholar]
- Ferguson, L.R.; Pearson, A.E. The clinical use of mutagenic anticancer drugs. Mutat. Res. Mol. Mech. Mutagen. 1996, 355, 1–12. [Google Scholar] [CrossRef]
- Les, F.; Prieto, J.M.; Arbonés-Mainar, J.; Valero, M.; López, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of intestinal absorption. J. Pharm. Sci. 1999, 88, 807–814. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



| Compounds | R1 | R2 | R3 | R4 | Yield (%) |
|---|---|---|---|---|---|
| 3a | H | H | H | H | 77 |
| 3b | OH | H | H | H | 94 |
| 3c | H | H | OMe | H | 86 |
| 3d | H | OMe | OH | H | 94 |
| 3e | H | OH | OMe | H | 93 |
| 3f | OH | H | H | NO2 | 85 |
| 3g | H | NO2 | H | H | 95 |
| 3h | H | H | N(Me)2 | H | 95 |
| 3i | H | OH | OH | H | 97 |
| 3j | H | OMe | OH | OMe | 91 |
| 4 | - | - | - | - | 86 |
| 5 | - | - | - | - | 90 |
| A. niger | C. albicans | S. cerevisiae | E. faecalis | E. coli | S. aureus | |
|---|---|---|---|---|---|---|
| MIC DMSO (%) IC50 NIC | 19.22 ± 1.3 4.36 ± 2.0 1.78 ± 0.4 | 14.98 ± 0.6 8.50 ± 0.5 4.94 ± 0.5 | 71.47 ± 1.7 35.62 ± 1.0 5.04 ± 1.1 | 20.02 ± 1.3 8.66 ± 0.6 3.68 ± 0.2 | 13.81 ± 3.5 16.36 ± 0.33 11.95 ± 2.9 | 32.64 ± 6.9 20.36 ± 5.2 8.52 ± 0.9 |
| 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i | 3j | 4 | 5 | GN | CP | VR | AP | PT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC A. niger IC50 NIC | NE | NE | NE | NE | NE | 32.6 ± 2.1 10.6 ± 0.8 3.4 ± 0.5 | NE | NE | NE | NE | NE | NE | ND | NA | 1.2 ± 0.05 0.2 ± 0.02 0.05 ± 0.01 | NA | NA |
| MIC C. albicans IC50 NIC | NE | 13.2 ± 1.6 5.8 ± 2.5 3.0 ± 2.2 | NE | NE | NE | 15.6 ± 0.9 10.4 ± 0.6 7.5 ± 1.1 | NE | NE | NE | NE | NE | NE | ND | NA | 29.7 ± 3.52 0.9 ± 0.07 0.00 ± 0.00 | NA | NA |
| MIC S. cerevisiae IC50 NIC | NE | NE | NE | NE | NE | 55.9 ± 6.2 17.9 ± 1.4 5.6 ± 0.9 | 179.2 ± 59.5 105.1 ± 22.1 63.7 ± 8.7 | NE | NE | NE | NE | 76.7 ± 5.7 22.8 ± 0.3 6.6 ± 0.7 | 93.7 ± 0.9 59.9 ± 0.9 44.5 ± 1.7 | NA | NA | NA | NA |
| MIC E. faecalis IC50 NIC | 32.3 ± 11.7 12.2 ± 3.3 3.6 ± 0.7 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NA | 1.15 ± 0.42 0.49 ± 0.07 0.30 ± 0.07 | NA | NA | NA |
| MIC S. aureus IC50 NIC | NE | NE | NE | NE | NE | 27.7 ± 4.7 9.5 ± 1.5 3.3 ± 1.5 | NE | NE | NE | NE | NE | NE | NA | 0.44 ± 0.09 0.10 ± 0.01 0.06 ± 0.02 | NA | NA | NA |
| MIC E. coli IC50 NIC | NE | 49.8 ± 5.0 12.1 ± 0.5 2.8 ± 0.5 | NE | 10.2 ± 0.0 3.3 ± 0.8 2.3 ± 0.1 | NE | 47.7 ± 1.0 15.8 ± 0.1 4.9 ± 0.2 | NE | NE | NE | NE | NE | 22.6 ± 14.1 12.4 ± 6.2 5.1 ± 2.4 | NA | 0.15 ± 0.009 0.07 ± 0.004 0.06 ± 0.003 | NA | NA | NA |
| MIC P. aeruginosa IC50 NIC | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NA | 0.47 ± 0.1 0.94 ± 0.0 ND | NA | NA | NA |
| MIC L. mexicana IC50 * NIC | NE | NE | NE | NE | NE | 5.3 ± 2.8 | NE | NE | NE | NE | NE | 81.5 ± 12.5 | NA | NA | NA | 0.18 ± 0.07 | 4.50 ± 0.46 |
| Entry | Compound | DPPH Scavenging Activity IC50 (µM) |
|---|---|---|
| 1 | 3a | >200 |
| 2 | 3b | NA |
| 3 | 3c | >200 |
| 4 | 3d | 28.33 ± 4.35 |
| 5 | 3e | >200 |
| 6 | 3f | >200 |
| 7 | 3g | >200 |
| 8 | 3h | 129.4 ± 18.7 |
| 9 | 3i | 18.9 ± 2.4 |
| 10 | 3j | 15.7 ± 3.2 |
| 11 | 4 | >200 |
| 12 | 5 | >200 |
| 13 | ascorbic acid | 14.5 ± 2.2 |
| 14 | quercetin | 7.3 ± 1.0 |
| 15 | caffeic acid | 16.2 ± 2.4 |
| Wt | sod1 | ||
|---|---|---|---|
| 3b | MIC IC50 NIC | 208.2 ± 4.0 192.0 ± 0.6 178.0 ± 2.1 | 164.1 ± 0.1 100.7 ± 4.2 53.3 ± 0.1 |
| 5 | MIC NIC IC50 | 65.2 ± 0.2 22.6 ± 0.6 8.1 ± 0.4 | 39.4 ± 0.9 19.5 ± 0.8 10.4 ± 1.1 |
| Entry | Compound | % Live Cells | µM | mg/mL |
|---|---|---|---|---|
| 1 | 3a | 27.1 ± 3.4 | 400 | 0.1165 |
| 2 | 3b | 19.3 ± 0.2 | 400 | 0.1229 |
| 3 | 3c | 26.1 ± 1.9 | 400 | 0.1285 |
| 4 | 3d | 17.6 ± 0.4 | 400 | 0.1349 |
| 5 | 3e | 27.1 ± 0.8 | 300 * | 0.1012 |
| 6 | 3f | 4.5 ± 1.4 | 400 | 0.1409 |
| 7 | 3g | 22.1 ± 0.6 | 300 * | 0.1009 |
| 8 | 3h | 20.4 ± 1.7 | 300 * | 0.1003 |
| 9 | 3i | 34.4 ± 2.4 | 400 | 0.1293 |
| 10 | 3j | 14.2 ± 0.6 | 400 | 0.1470 |
| 11 | 4 | 23.8 ± 3.4 | 400 | 0.1125 |
| 12 | 5 | 2.7 ± 0.5 | 400 | 0.1270 |
| 13 | Saponine ** | IC50 (50%) | NA | 0.1410 ± 0.02 |
| Compound | MW | HBA | HBD | nrotb | PSA | M | T | R | I | cLogP | cLogS | DL | DS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3a | 291.35 | 4 | 0 | 3 | 35.91 | high | NE | NE | NE | 2.16 | −3.24 | 4.25 | 0.52 |
| 3b | 307.35 | 5 | 1 | 3 | 56.14 | high | NE | NE | NE | 1.81 | −2.94 | 4.25 | 0.53 |
| 3c | 321.38 | 5 | 0 | 4 | 45.14 | high | NE | NE | NE | 2.09 | −3.26 | 4.24 | 0.51 |
| 3d | 337.38 | 6 | 1 | 4 | 65.37 | high | low | NE | NE | 1.74 | −2.96 | 4.24 | 0.41 |
| 3e | 337.38 | 6 | 1 | 4 | 65.37 | high | NE | NE | NE | 1.74 | −2.96 | 4.24 | 0.52 |
| 3f | 352.35 | 8 | 1 | 4 | 101.96 | high | NE | NE | NE | 0.67 | −3.40 | −0.85 | 0.33 |
| 3g | 336.35 | 7 | 0 | 4 | 81.73 | high | NE | NE | NE | 1.01 | −3.70 | −0.85 | 0.32 |
| 3h | 334.42 | 5 | 0 | 4 | 39.15 | high | high | NE | NE | 2.05 | −3.28 | 4.56 | 0.30 |
| 3i | 323.35 | 6 | 2 | 3 | 76.37 | high | NE | NE | NE | 1.47 | −2.65 | 4.25 | 0.53 |
| 3j | 367.40 | 7 | 1 | 5 | 74.60 | high | NE | NE | NE | 1.67 | −2.98 | 4.24 | 0.50 |
| 4 | 281.31 | 5 | 0 | 3 | 49.05 | high | NE | NE | NE | 1.35 | −2.92 | 4.08 | 0.54 |
| 5 | 317.39 | 4 | 0 | 4 | 35.91 | high | NE | NE | NE | 2.18 | −3.53 | 4.28 | 0.50 |
| VR | 349.32 | 6 | 1 | 5 | 76.72 | NE | NE | NE | NE | 1.48 | −3.23 | 4.08 | 0.84 |
| CP | 331.35 | 6 | 2 | 3 | 72.88 | NE | NE | NE | NE | −1.53 | −3.32 | 2.05 | 0.82 |
| Entry | Compound | GPCR | ICM | KI | NRL | PI | EI |
|---|---|---|---|---|---|---|---|
| 1 | 3a | −0.90 | −1.12 | −0.63 | −1.08 | −1.07 | −0.53 |
| 2 | 3b | −0.82 | −1.14 | −0.58 | −0.92 | −0.95 | −0.48 |
| 3 | 3c | −0.85 | −1.10 | −0.60 | −0.98 | −1.00 | −0.54 |
| 4 | 3d | −0.79 | −1.03 | −0.54 | −0.90 | −0.98 | −0.47 |
| 5 | 3e | −0.79 | −1.03 | −0.54 | −0.90 | −0.98 | −0.47 |
| 6 | 3f | −0.88 | −1.07 | −0.65 | −0.91 | −0.96 | −0.55 |
| 7 | 3g | −0.93 | −1.05 | −0.69 | −1.03 | −1.05 | −0.59 |
| 8 | 3h | −0.76 | −1.01 | −0.51 | −0.91 | −0.92 | −0.48 |
| 9 | 3i | −0.77 | −1.01 | −0.54 | −0.86 | −0.95 | −0.44 |
| 10 | 3j | −0.75 | −0.96 | −0.49 | −0.86 | −0.89 | −0.41 |
| 11 | 4 | −1.01 | −1.26 | −0.96 | −1.35 | −1.30 | −0.70 |
| 12 | 5 | −0.63 | −0.84 | −0.75 | −0.93 | −0.96 | −0.51 |
| 13 | VRC | 0.23 | 0.17 | 0.14 | −0.22 | 0.02 | 0.19 |
| 14 | CP | 0.12 | −0.04 | −0.07 | −0.19 | −0.20 | 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teran, R.; Guevara, R.; Mora, J.; Dobronski, L.; Barreiro-Costa, O.; Beske, T.; Pérez-Barrera, J.; Araya-Maturana, R.; Rojas-Silva, P.; Poveda, A.; et al. Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine. Molecules 2019, 24, 2696. https://doi.org/10.3390/molecules24152696
Teran R, Guevara R, Mora J, Dobronski L, Barreiro-Costa O, Beske T, Pérez-Barrera J, Araya-Maturana R, Rojas-Silva P, Poveda A, et al. Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine. Molecules. 2019; 24(15):2696. https://doi.org/10.3390/molecules24152696
Chicago/Turabian StyleTeran, Rommy, Rommel Guevara, Jessica Mora, Lizeth Dobronski, Olalla Barreiro-Costa, Timo Beske, Jorge Pérez-Barrera, Ramiro Araya-Maturana, Patricio Rojas-Silva, Ana Poveda, and et al. 2019. "Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine" Molecules 24, no. 15: 2696. https://doi.org/10.3390/molecules24152696
APA StyleTeran, R., Guevara, R., Mora, J., Dobronski, L., Barreiro-Costa, O., Beske, T., Pérez-Barrera, J., Araya-Maturana, R., Rojas-Silva, P., Poveda, A., & Heredia-Moya, J. (2019). Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine. Molecules, 24(15), 2696. https://doi.org/10.3390/molecules24152696