Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Cur-NEs
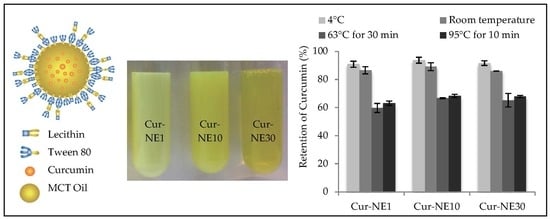
2.2. Morphology of Cur-NEs
2.3. Physical Stability of Cur-NEs Under Processing Conditions
2.4. Physicochemical Stability of Cur-NEs Under Storage Conditions
2.5. Chemical Stability of Cur-NEs Under Thermal Processing and Storage Conditions
2.6. Lipid Oxidation Inhibition of Cur-NEs Fortified with Milk
2.7. Color Change of Fortified Milk
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Curcumin Nanoemulsions (Cur-NEs)
3.3. Characterization and Morphology of Cur-NEs
3.4. Determination of Encapsulation Efficiency (%EE) and Curcumin Concentration
3.5. Stability of Cur-NEs Under Processing Conditions
3.6. Stability of Cur-NEs Under Storage Conditions
3.7. Lipid Oxidation Inhibition by Cur-NEs
3.8. Color Measurement
3.9. Data Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jayaprakasha, G.K.; Jaganmohan Rao, L.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; pp. 105–125. [Google Scholar] [CrossRef]
- Carvalho, D.d.M.; Takeuchi, K.P.; Geraldine, R.M.; Moura, C.J.d.; Torres, M.C.L. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Jin, H.H.; Lu, Q.; Jiang, J.G. Curcumin liposomes prepared with milk fat globule membrane phospholipids and soybean lecithin. J. Dairy Sci. 2016, 99, 1780–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Giménez, P.J.; Fernández-López, J.A.; Angosto, J.M.; Obón, J.M. Comparative Thermal Degradation Patterns of Natural Yellow Colorants Used in Foods. Plant Foods Hum. Nutr. 2015, 70, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Manjunatha, H.; Srinivasan, K. Effect of heat processing of spices on the concentrations of their bioactive principles: Turmeric (Curcuma longa), red pepper (Capsicum annuum) and black pepper (Piper nigrum). J. Food Compos. Anal. 2007, 20, 346–351. [Google Scholar] [CrossRef]
- Suresh, D.; Gurudutt, K.N.; Srinivasan, K. Degradation of bioactive spice compound: Curcumin during domestic cooking. Eur. Food Res. Technol. 2009, 228, 807–812. [Google Scholar] [CrossRef]
- Saifullah, M.; Ahsan, A.; Shishir, M.R.I. Production, stability and application of micro- and nanoemulsion in food production and the food processing industry. In Emulsions; Academic Press: Cambridge, MA, USA, 2016; pp. 405–442. [Google Scholar]
- Joung, H.J.; Choi, M.J.; Kim, J.T.; Park, S.H.; Park, H.J.; Shin, G.H. Development of Food—Grade Curcumin Nanoemulsion and its Potential Application to Food Beverage System: Antioxidant Property and In Vitro Digestion. J. Food Sci. 2016, 81, N745–N753. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Chuacharoen, T.; Sabliov, C.M. Supplement Delivery at the Nanoscale. In Nanotechnologies in Food, 2nd ed.; The Royal Society of Chemistry: London, UK, 2017; pp. 97–117. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef] [PubMed]
- Haidar, I.; Harding, I.H.; Bowater, I.C.; Eldridge, D.S.; Charman, W.N. The role of lecithin degradation on the pH dependent stability of halofantrine encapsulated fat nano-emulsions. Int. J. Pharm. 2017, 528, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wu, J.; Zhong, Q. Eugenol improves physical and chemical stabilities of nanoemulsions loaded with β-carotene. Food Chem. 2016, 194, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Guttoff, M.; Saberi, A.H.; McClements, D.J. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: Factors affecting particle size and stability. Food Chem. 2015, 171, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Yan, H.H.; Chen, Z.Q.; He, M. Effects of emulsifier type and environmental stress on the stability of curcumin emulsion. J. Dispers. Sci. Technol. 2017, 38, 1375–1380. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Formation of Flavor Oil Microemulsions, Nanoemulsions and Emulsions: Influence of Composition and Preparation Method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef]
- Chuah, A.M.; Kuroiwa, T.; Kobayashi, I.; Nakajima, M. Effect of chitosan on the stability and properties of modified lecithin stabilized oil-in-water monodisperse emulsion prepared by microchannel emulsification. Food Hydrocoll. 2009, 23, 600–610. [Google Scholar] [CrossRef]
- Kim, S.H.; Ji, Y.S.; Lee, E.S.; Hong, S.T. Ostwald Ripening Stability of Curcumin-Loaded MCT Nanoemulsion: Influence of Various Emulsifiers. Prev. Nutr. Food Sci. 2016, 21, 289–295. [Google Scholar] [CrossRef] [Green Version]
- King, R.L. Oxidation of Milk Fat Globule Membrane Material. I. Thiobarbituric Acid Reaction as a Measure of Oxidized Flavor in Milk and Model Systems. J. Dairy Sci. 1962, 45, 1165–1171. [Google Scholar] [CrossRef]
- Spanier, A.M.; Traylor, R.D. A rapid, direct chemical assay for the quantitative determination of thiobarbituric acid reactive substances in raw, cooked, and cooked/stored muscle foods. J. Muscle Foods 1991, 2, 165–176. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. The potential of zein nanoparticles to protect entrapped β-carotene in the presence of milk under simulated gastrointestinal (GI) conditions. LWT Food Sci. Technol. 2016, 72, 302–309. [Google Scholar] [CrossRef]
- Fenaille, F.; Mottier, P.; Turesky, R.J.; Ali, S.; Guy, P.A. Comparison of analytical techniques to quantify malondialdehyde in milk powders. J. Chromatogr. A 2001, 921, 237–245. [Google Scholar] [CrossRef]
- Popov-Raljić, J.V.; Lakić, N.S.; Laličić-Petronijević, J.G.; Barać, M.B.; Sikimić, V.M. Color Changes of UHT Milk During Storage. Sensors 2008, 8, 5961–5974. [Google Scholar] [CrossRef] [Green Version]
- Kneifel, W.; Ulberth, F.; Schaffer, E. Tristimulus colour reflectance measurement of milk and dairy products. Le Lait 1992, 72, 383–391. [Google Scholar] [CrossRef]
- Jrad, Z.; Girardet, J.M.; Adt, I.; Oulahal, N.; Degraeve, P.; Khorchani, T.; El Hatmi, H. Antioxidant activity of camel milk casein before and after in vitro simulated enzymatic digestion. Mljekarstvo 2014, 64, 287–294. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Cur-NE1 | Cur-NE10 | Cur-NE30 | |
|---|---|---|---|
| Composition ratio (%) | |||
| MCT | 10 | 10 | 10 |
| Lecithin | 0.18 | 1.8 | 5.4 |
| Tween 80 | 0.12 | 1.2 | 3.6 |
| Characteristics | |||
| Size (nm) | 193.93 ± 12.63 | 86.68 ± 9.58 | 44.39 ± 3.11 |
| PDI (a.u.) | 0.18 ± 0.09 | 0.19 ± 0.07 | 0.23 ± 0.02 |
| Zeta Potential (mV) | −62.93 ± 3.22 | −54.27 ± 3.90 | −48.00 ± 2.83 |
| EE (%) | 92.86 ± 0.64 | 95.38 ± 1.56 | 99.51 ± 0.75 |
| Cur-NE1 | Cur-NE10 | Cur-NE30 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Size (nm) | PDI (a.u) ns | Zeta (mV) | Size (nm) ns | PDI (a.u) ns | Zeta (mV) | Size (nm) ns | PDI (a.u) | Zeta (mV) | |
| Control | 193.9 ± 12.6 a | 0.18 ± 0.09 | −62.9 ± 3.2 a | 86.7 ± 9.6 | 0.19 ± 0.07 | −54.3 ± 3.9 a | 44.4 ± 3.1 | 0.23 ± 0.02 a | −48.0 ± 2.8 a |
| pH | |||||||||
| 2 | 197.3 ± 0.9 | 0.19 ± 0.00 | −7.5 ± 0.1 d | 91.2 ± 2.1 | 0.16 ± 0.02 | −37.2 ± 3.0 b | 49.7 ± 0.3 | 0.29 ± 0.01 a | −23.8 ± 1.6 c |
| 7 | 191.9 ± 5.8 | 0.20 ± 0.00 | −24.9 ± 4.6 c | 90.9 ± 2.7 | 0.16 ± 0.00 | −51.6 ± 1.1 a | 46.9 ± 0.8 | 0.25 ± 0.02 a | −43.6 ± 0.1 a |
| 9 | 204.4 ± 4.2 | 0.16 ± 0.01 | −51.9 ± 1.4 b | 90.6 ± 2.3 | 0.17 ± 0.01 | −50.2 ± 4.4 a | 43.9 ± 2.5 | 0.28 ± 0.00 a | −35.5 ± 6.8 b |
| Ionic strength | |||||||||
| 0.1 M NaCl | 282.8 ± 6.9 c | 0.22 ± 0.02 | −2.1 ± 0.3 d | 88.7 ± 1.7 | 0.16 ± 0.02 | −8.7 ± 0.2 c | 45.7 ± 1.7 | 0.18 ± 0.01 c | −6.4 ± 0.5 c |
| 0.5 M NaCl | 292.5 ± 5.3 c | 0.23 ± 0.03 | −5.1 ± 0.2 d | 86.9 ± 0.4 | 0.13 ± 0.01 | −14.2 ± 1.0 c | 44.4 ± 2.7 | 0.18 ± 0.03 c | −9.4 ± 0.9 c |
| 1.0 M NaCl | 220.2 ± 7.8 b | 0.20 ± 0.00 | −21.3 ± 1.2 c | 83.7 ± 2.9 | 0.14 ± 0.00 | −25.2 ± 0.1 b | 43.9 ± 2.0 | 0.22 ± 0.02 a | −18.9 ± 2.8 b |
| Thermal processing | |||||||||
| 63 °C (30 min) | 197.3 ± 0.6 | 0.19 ± 0.01 | −17.5 ± 0.1 c | 81.1 ± 4.7 | 0.21 ± 0.00 | −48.4 ± 1.1 | 49.2 ± 0.9 | 0.39 ± 0.01 b | −43.8 ± 1.6 a |
| 95 °C (10 min) | 190.9 ± 5.9 | 0.20 ± 0.00 | −14.9 ± 4.6 c | 80.9 ± 5.5 | 0.22 ± 0.02 | −50.3 ± 0.1 | 46.9 ± 0.8 | 0.42 ± 0.02 b | −43.6 ± 0.1 a |
| Conditions | Cur-NE1 | Cur-NE10 | Cur-NE30 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Size (nm) | PDI (a.u) | Zeta (mV) | Size (nm) | PDI (a.u) ns | Zeta (mV) | Size (nm) ns | PDI (a.u) ns | Zeta (mV) | |
| Day 0 (control) | 193.9 ± 12.6 a | 0.18 ± 0.09 a | −62.9 ± 3.2 a | 86.7 ± 9.6 a | 0.19 ± 0.07 | −54.3 ± 3.9 a | 44.4 ± 3.1 | 0.23 ± 0.02 | −48.0 ± 2.8 a |
| Day 15 | |||||||||
| 4 (°C) | 211.2 ± 5.1 b | 0.08 ± 0.00 b | −51.4 ± 3.1 b | 83.5 ± 5.07 a | 0.16 ± 0.01 | −50.5 ± 2.1 a | 46.6 ± 2.5 | 0.28 ± 0.02 | −43.6 ± 5.6 a |
| RT (°C) | 211.4 ± 9.3 b | 0.08 ± 0.01 b | −47.3 ± 0.2 b | 110.7 ± 8.6 b | 0.20 ± 0.03 | −35.9 ± 1.1 b | 47.1 ± 0.1 | 0.25 ± 0.05 | −20.1 ± 1.8 b |
| Samples | TBARS (mg/L) | ||
|---|---|---|---|
| Day 0 | Day 5 | Day 15 | |
| Control (milk) | 1.369 ± 0.020 a,A | 0.978 ± 0.014 a,B | 0.642 ± 0.027 a,C |
| Cur-NE1 | 0.297 ± 0.042 b,A | 0.101 ± 0.029 b,B | 0.069 ± 0.019 b,C |
| Cur-NE10 | 0.209 ± 0.041 c,A | 0.087 ± 0.019 c,B | 0.028 ± 0.002 c,C |
| Cur-NE30 | 0.095 ± 0.035 d,A | 0.029 ± 0.039 d,B | 0.013 ± 0.001 c,B |
| Samples | Color Parameters (CIE L*, a*, b* System) | ||||
|---|---|---|---|---|---|
| L* | a* | b* | Chroma, C* | Hue, h* | |
| Milk (control) | |||||
| Day 0 | 89.60 ± 0.17 a,A | −3.02 ± 0.01 a,A | 10.22 ± 0.03 a,A | 10.66 ± 0.03 a,A | 106.46 ± 0.16 a,A |
| Day 5 | 89.57 ± 0.01 a,A | −3.06 ± 0.03 a,A | 10.18 ± 0.02 a,A | 10.63 ± 0.02 a,A | 106.73 ± 0.30 a,A |
| Day 15 | 89.32 ± 0.02 a,B | −3.22 ± 0.09 a,B | 9.16 ± 0.43 a,B | 9.71 ± 0.40 a,B | 109.37 ± 0.54 a,B |
| Cur-NE1 | |||||
| Day 0 | 89.65 ± 0.01 a,A | −3.03 ± 0.04 a,A | 10.54 ± 0.03 b,A | 10.97 ± 0.03 b,A | 106.04 ± 0.36 a,A |
| Day 5 | 89.55 ± 0.02 a,B | −3.06 ± 0.02 a,A | 10.37 ± 0.03 b,B | 10.81 ± 0.03 b,B | 106.54 ± 0.23 a,A |
| Day 15 | 89.52 ± 0.03 b,B | −3.23 ± 0.01 a,B | 10.04 ± 0.02 b,C | 10.55 ± 0.02 b,C | 107.83 ± 0.16 b,B |
| Cur-NE10 | |||||
| Day 0 | 89.66 ± 0.01 a,A | −2.99 ± 0.02 a,A | 10.57 ± 0.01 b,A | 10.98 ± 0.01 b,A | 105.79 ± 0.13 b,A |
| Day 5 | 89.55 ± 0.02 a,B | −3.04 ± 0.02 a,A | 10.36 ± 0.05 b,B | 10.80 ± 0.05 b,B | 106.35 ± 0.40 b,B |
| Day 15 | 89.53 ± 0.01 b,B | −3.21 ± 0.03 a,B | 10.05 ± 0.02 b,C | 10.55 ± 0.02 b,C | 107.71 ± 0.22 b,C |
| Cur-NE30 | |||||
| Day 0 | 89.70 ± 0.01 a,A | −2.95 ± 0.01 a,A | 10.63 ± 0.03 c,A | 11.03 ± 0.03 b,A | 105.51 ± 0.30 b,A |
| Day 5 | 89.58 ± 0.01 a,B | −3.04 ± 0.02 a,A | 10.42 ± 0.04 c,B | 10.85 ± 0.04 b,B | 106.26 ± 0.24 b,B |
| Day 15 | 89.56 ± 0.03 b,B | −3.19 ± 0.02 a,B | 10.09 ± 0.02 b,C | 10.58 ± 0.02 b,C | 107.54 ± 0.12 b,C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules 2019, 24, 2744. https://doi.org/10.3390/molecules24152744
Chuacharoen T, Prasongsuk S, Sabliov CM. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules. 2019; 24(15):2744. https://doi.org/10.3390/molecules24152744
Chicago/Turabian StyleChuacharoen, Thanida, Sehanat Prasongsuk, and Cristina M. Sabliov. 2019. "Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization" Molecules 24, no. 15: 2744. https://doi.org/10.3390/molecules24152744
APA StyleChuacharoen, T., Prasongsuk, S., & Sabliov, C. M. (2019). Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules, 24(15), 2744. https://doi.org/10.3390/molecules24152744