Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass
Abstract
:1. Introduction
2. Results and Discussion
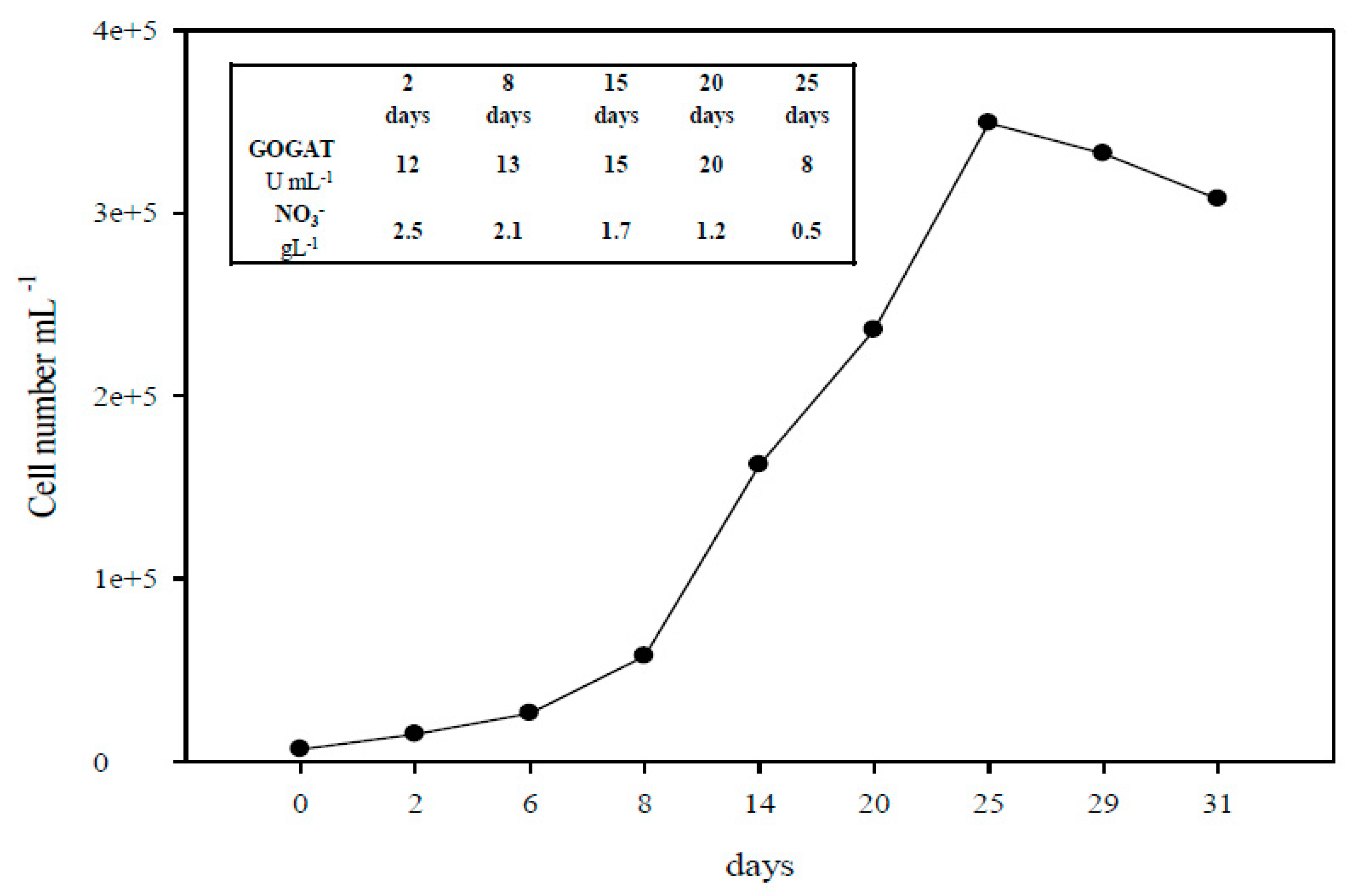
2.1. The Monitoring of A. platensis Cultivation
2.2. Influence of Different Storage Methods on Photosynthetic Pigments of A. platensis
2.3. Total Proteins and Antioxidant Molecules in A. platensis Samples
2.4. Phytochemical Screening and Antioxidant Activity
3. Materials and Methods
3.1. Chemicals
3.2. Culture Conditions for A. platensis and Application of Drying Methods
3.3. Protein Determination
3.4. Phycobiliproteins
3.5. Chlorophyll and Carotenoids
3.6. Ascorbic and Dehydroascorbic Acid
3.7. Total Phenol and Total Flavonoid Analysis
3.8. Antioxidant Activity Assays
3.9. Phenolic Profile Identification
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and medical applications of spirulina microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, K.S.; Yang, T.J.; Hwang, J.H.; Chan, Y.C.; Lee, I.T. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012, 15, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A. Spirulina: Growth, physiology and biochemistry. In Spirulina Platensis Arthrospira: Physiology, Cell-Biology and Biotechnology; Taylor & Francis Ltd.: London, UK, 1997; pp. 43–65. [Google Scholar]
- Furmaniak, M.A.; Misztak, A.E.; Franczuk, M.D.; Wilmotte, A.; Waleron, M.; Waleron, K.F. Edible cyanobacterial genus Arthrospira: Actual state of the art in cultivation methods, genetics, and application in medicine. Front. Microbiol. 2017, 8, 2541. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Mata, L.; Wang, N.; Zhao, J.; de Nys, R.; Paul, N.A. Manipulating antioxidant content in macroalgae in intensive land-based cultivation systems for functional food applications. Algal Res. 2015, 8, 153–160. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Jiménez, C.; Figueroa, F.L.; Niell, F.X. Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J. Appl. Phycol. 1999, 10, 461–469. [Google Scholar] [CrossRef]
- Zachleder, V.; Brányiková, I. Starch overproduction by means of algae. In Algal Biorefineries; Bajpai, R., Prokop, A., Zappi, M., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 217–240. [Google Scholar]
- Tzovenis, I.; de Pauw, N.; Sorgeloos, P. Optimisation of T-ISO biomass production rich in essential fatty acids: I. Effect of different light regimes on growth and biomass production. Aquaculture 2003, 216, 203–222. [Google Scholar] [CrossRef]
- El-Baky, H.H.A.; El Baz, F.K.; El-Baroty, G.S. Characterization of nutraceutical compounds in blue green alga Spirulina maxima. J. Med. Plants Res. 2008, 2, 292–300. [Google Scholar]
- El-Baky, H.H.A.; El Baz, F.K.; El-Baroty, G.S. Production of phenolic compounds from Spirulina maxima microalgae and its protective effects. Afr. J. Biotechnol. 2009, 24, 7059–7067. [Google Scholar]
- Ismaiel, M.M.S.; El-Ayouty, Y.M.; Piercey-Normorea, M. Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef]
- Dhiab, R.B.; Ouada, H.B.; Boussetta, H.; Franck, F.; Elabed, A.; Brouers, M. Growth, fluorescence, photosynthetic O2 production and pigment content of salt adapted cultures of Arthrospira (Spirulina) platensis. J. Appl. Phycol. 2007, 19, 293–301. [Google Scholar] [CrossRef]
- Ürek, R.Ö.; Tarhan, L. The relationship between the antioxidant system and phycocyanin production in Spirulina maxima with respect to nitrate concentration. Turk. J. Bot. 2012, 36, 369–377. [Google Scholar]
- Morgunov, I.G.; Karpukhina, O.V.; Kamzolova, S.V.; Samoilenko, V.A.; Inozemtsev, A.N. Investigation of the effect of biologically active threo-Ds-isocitric acid on oxidative stress in Paramecium caudatum. Prep. Biochem. Biotechnol. 2018, 48, 1–5. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Karpukhina, O.V.; Bokieva, S.B.; Inozemtsev, A.N. Biosynthesis of isocitric acid in repeated-batch culture and testing of its stress-protective activity. Appl. Microbiol. Biotechnol. 2019, 103, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Ratti, C. Hot air and freeze-drying of high-values foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Pak Dek, M.S.; Hairuddin, M.R. Effect of freeze-drying on the antioxidant compounds and antioxidant activity of selected tropical fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef]
- Rahman, M.M.; Das, R.; Hoque, M.M.; Zzaman, W. Effect of freeze drying on antioxidant activity and phenolic contents of mango (Mangifera indica). Int. Food Res. J. 2015, 22, 613–617. [Google Scholar]
- Chouha, S.; Kaitwas, V.; Kachouli, R.; Barghava, S. Productivity of the cyanobacterium Spirulina platensis in cultures using high bicarbonate and different nitrogen sources. Am. J. Plant Physiol. 2013, 8, 17–31. [Google Scholar]
- Malapascua, J.R.F.; Jerez, C.G.; Sergejevová, M.; Figueroa, M.F.; Masojídek, J. Photosynthesis monitoring to optimize growth of microalgal mass cultures: Application of chlorophyll fluorescence techniques. Aquat. Biol. 2014, 22, 123–140. [Google Scholar] [CrossRef]
- Hidasi, N.; Belay, A. Diurnal variation of various culture and biochemical parameters of Arthrospira platensis in large-scale outdoor raceway ponds. Algal Res. 2018, 29, 121–129. [Google Scholar] [CrossRef]
- Deschoenmaeker, F.; Facchini, R.; Leroy, B.; Badri, H.; Zhang, C.C.; Wattiez, R. Proteomic and cellular views of Arthrospira sp. PCC 8005 adaptation to nitrogen depletion. Microbiology 2014, 160, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liang, N.; Shi, A.; Liu, L.; Chen, J.; Du, G. Enhanced of α-ketoglutarate production in Torulopsis glabrata: Redistribution of carbon flux from pyruvate to α-ketoglutarate. Biotechnol. Bioprocess Eng. 2009, 14, 134–139. [Google Scholar] [CrossRef]
- Martelli, G.; Folli, C.; Visai, L.; Daglia, M.; Ferrari, D. Thermal stability improvement of blue colorant C-Phycocyanin from Spirulina platensis for food industry applications. Process Biochem. 2014, 49, 154–159. [Google Scholar] [CrossRef]
- Oliveira, E.G.; Rosa, G.S.; Moraes, M.A.; Pinto, L.A.A. Phycocyanin content of Spirulina platensis dried in spouted bed and thin layer. J. Food Process. Eng. 2008, 31, 34–50. [Google Scholar] [CrossRef]
- Sidari, R.; Tofalo, R. A comprehensive overview on microalgal-fortified/based food and beverages. Food Rev. Int. 2019, 1–29. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abuzead, S.M.; Halawa, S.M. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS ONE 2013, 8, e72991. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, H.Y. Effect of ultrasonic extraction on production and structural changes of C-phycocyanin from marine Spirulina maxima. Int. J. Mol. Sci. 2018, 19, 220. [Google Scholar] [CrossRef]
- Böckera, L.; Ortmanna, S.; Surbera, J.; Leebb, E.; Reinekeb, K.; Mathys, A. Biphasic short time heat degradation of the blue microalgae protein phycocyanin from Arthrospira platensis. Innov. Food Sci. Emer. Technol. 2019, 52, 116–121. [Google Scholar] [CrossRef]
- Cherdkiatikul, T.; Suwanwong, Y. Production of the α and β Subunits of Spirulina Allophycocyanin and C-Phycocyanin in Escherichia coli: A comparative study of their antioxidant activities. J. Biomol. Screen. 2014, 19, 959–965. [Google Scholar] [CrossRef]
- Sujatha, K.; Nagarajan, P. Optimization of growth conditions for carotenoid production from Spirulina platensis (Geitler). Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 325–328. [Google Scholar]
- Gaese, H. Chemical composition and potential application of Spirulina platensis biomass. Int. J. Agr. Envir. 2012, 4, 35–40. [Google Scholar]
- Ferreira, J.E.; Rodriguez-Amaya, D.B. Degradation of lycopene and beta-carotene in model systems and in lyophilized guava during ambient storage: Kinetics, structure, and matrix effects. J. Food Sci. 2008, 73, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Villota, R.; Saguy, I.; Karel, M. Storage stability of dehydrated food evaluation of literature data. J. Food Qual. 1980, 3, 123–216. [Google Scholar] [CrossRef]
- Santos, P.H.S.; Silva, M.A. Retention of vitamin C in drying processes of fruits and vegetables—A review. Dry. Technol. 2008, 26, 1421–1437. [Google Scholar] [CrossRef]
- Stešková, A.; Morochovičová, M.; Lešková, E. Vitamin C degradation during storage of fortified foods. J. Food Nutr. Res. 2006, 45, 55–61. [Google Scholar]
- Papalia, T.; Barreca, D.; Panuccio, M.R. Assessment of antioxidant and cytoprotective potential of jatropha (Jatropha curcas) grown in Southern Italy. Int. J. Mol. Sci. 2017, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Onofrejova, L.; Vasickova, J.; Klejdusa, B.; Stratil, P.; Misurcova, L.; Kracmar, S.; Kopecky, J.; Vacek, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Kopecký, J.; Benešová, L.; Vacek, J. Solid-phase/supercritical-fluid extraction for liquid chromatography of phenolic compounds in freshwater microalgae and selected cyanobacterial species. J. Chromatogr. A 2009, 1216, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, M.S. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Isoflavone Content of Selected Foods; Release 2.0.; USDA: Bethesda, MD, USA, 2008; pp. 1–69. [Google Scholar]
- Polkowski, K.; Mazurek, A.P. Biological properties of genistein: A review of in vitro and in vivo data. Acta Pol. Pharm. 2000, 57, 135–155. [Google Scholar] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. Genistein and phycocyanobilin may prevent hepatic fibrosis by suppressing proliferation and activation of hepatic stellate cells. Med. Hypotheses 2009, 72, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Z.; Sun, P.; Chen, T.; Zhang, J. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–415. [Google Scholar]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, C. Contribution à L’étude d’une Cyanophycée. Influence de Divers facteurs Physiques et Chimiques sur la Croissance et la Photosynthese de Spirulina maxima Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1996. [Google Scholar]
- Colla, L.M.; Reinehr, C.O.; Reichert, C.J.; Costa, A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Biores. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Panuccio, M.R.; Chaabani, S.; Roula, R.; Muscolo, A. Bio-priming mitigates detrimental effects of salinity on maize improving antioxidant defense and preserving photosynthetic efficiency. Plant Physiol. Biochem. 2018, 132, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Sidari, M.; Panuccio, M.R.; de Sanctis, C.; Finocchiaro, A. Early effects of phenolic compounds, extracted from two forest litters, on ammonium uptake and assimilation in Pinus laricio and Pinus pinaster seedlings. Plant Soil 2005, 269, 309–320. [Google Scholar] [CrossRef]
- Payne, J.; Stewart, J. The chemical composition of the thallus wall of Characiosophon rivularis (Characiosiphonaceae, Chlorophyta). Phycologia 1988, 27, 43–49. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bennett, A.; Bogard, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Filament Biol. 1973, 58, 419–438. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Fazio, A.; Papalia, T.; Barreca, D. Antioxidant properties and flavonoid profile in leaves of Calabrian Lavandula multifida L., an autochthon plant of Mediterranean Southern regions. Chem. Biodiv. 2016, 13, 416–421. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337. [Google Scholar] [CrossRef]
- Seal, T. Qantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharm. Sci. 2016, 6, 157–166. [Google Scholar] [CrossRef]


| Biomolecules | A. platensis Fresh | A. platensis Frozen | A. platensis Oven-Dried | A. platensis Freeze-Dried |
|---|---|---|---|---|
| C-Phycocyanin (mg g D.W.−1) | 44.81 ± 0.35b | 50.21 ± 0.74a | 18.88 ± 0.35d | 38.03 ± 0.50c |
| Allophycocyanin (mg g D.W.−1) | 18.90 ± 1.03a | 4 ± 0.09b | 4.52 ± 0.51b | 5.48 ± 0.16b |
| Phycoerythrin (mg g D.W.−1) | 5.28 ± 0.16a | 0.99 ± 0.25c | 1.47 ± 0.26c | 2.17 ± 0.11b |
| Chlorophyll a (mg g D.W.−1) | 12.45 ± 0.04a | 9.95 ± 0.09b | 1.47 ± 0.03d | 6.44 ± 0.03c |
| Carotenoids (mg g D.W.−1) | 3.82 ± 0.15a | 3.31 ± 0.05b | 0.90 ± 0.001d | 2.22 ± 0.004c |
| Biomolecules | A. platensis Fresh | A. platensis Frozen | A. platensis Oven-Dried | A. platensis Freeze-Dried |
|---|---|---|---|---|
| Total Proteins (mg g D.W. −1) | 188.60 ± 13.55b | 283.96 ± 11.79a | 122.73 ± 2.53c | 167.09 ± 4.35b |
| Ascorbic acid (mg g D.W.−1) | 1678.292.62b | 3149.54 ± 7.99a | 354.79 ± 0.93d | 1403.9 ± 11.49c |
| Dehydroascorbic acid (mg g D.W.−1) | 1998.99 ± 7.01c | 1362.93 ± 22.78d | 3296.69 ± 15.56b | 4660.74 ± 34.56a |
| Total Phenols (mg g D.W.−1) | 15.77 ± 1.10b | 22.65 ± 0.46a | 12.14 ± 1.84c | 11.91 ± 0.28c |
| Total Flavonoids (mg g D.W.−1) | 20.82 ± 0.08b | 8.04 ± 0.29c | 4.31 ± 0.11d | 30.92 0.17a |
| Compounds | Rt | A. platensis Fresh | A. platensis Oven-Dried | A. platensis Frozen | A. platensis Freeze-Dried |
|---|---|---|---|---|---|
| Gallic acid | 6.11 | + | + | + | + |
| Catechin | 11.28 | + | + | + | - |
| Caffeic acid | 13.22 | + | - | + | - |
| p-Hydroxybenzoic acid | 14.13 | + | + | + | - |
| p-Cumaric acid | 18.69 | + | + | - | + |
| Ferulic acid | 18.81 | + | + | - | + |
| Quercetin | 29.59 | + | - | + | + |
| Genistein | 34.95 | + | + | + | + |
| Kaempferol | 36.67 | + | + | + | + |
| Biomass | C-PC | CAROT | CHL | PHEN | FLAVON | ASC | |
|---|---|---|---|---|---|---|---|
| DPPH | Fresh | ||||||
| r | −0.994 | 0.879 | −0.736 | 0.546 | 0.147 | −0.087 | |
| R2 | 0.989 | 0.77 | 0.536 | n.s. | n.s. | n.s. | |
| Frozen | |||||||
| r | −0.099 | −0.13 | −0.805 | −1.00 | −0.528 | −0.926 | |
| R2 | n.s. | n.s. | 0.648 | 0.999 | n.s. | 0.857 | |
| Oven-dried | |||||||
| r | 0.624 | 0.776 | −0.928 | −0.586 | −0.863 | 0.541 | |
| R2 | n.s. | 0.603 | 0.861 | n.s. | 0.745 | n.s. | |
| Freeze-dried | |||||||
| r | 0.961 | 0.926 | 0.895 | 0.676 | 0.977 | 0.709 | |
| R2 | 0.924 | 0.858 | 0.801 | n.s. | 0.954 | 0.502 | |
| TAC | Fresh | ||||||
| r | 0.207 | 0.385 | −0.604 | 0.778 | 0.969 | −0.982 | |
| R2 | n.s. | n.s. | n.s. | 0.606 | 0.939 | 0.965 | |
| Frozen | |||||||
| r | 0.084 | 0.116 | 0.796 | 1.00 | 0.516 | 0.920 | |
| R2 | n.s. | n.s. | 0.634 | 1.00 | n.s. | 0.847 | |
| Oven-dried | |||||||
| r | 0.571 | −0.999 | 0.516 | 0.956 | 0.993 | 0.938 | |
| R2 | n.s. | 0.999 | n.s. | 0.913 | 0.986 | 0.88 | |
| Freeze-dried | |||||||
| r | −0.934 | 0.899 | 0.863 | 0.627 | 0.960 | 0.66 | |
| R2 | 0.872 | 0.808 | 0.745 | n.s. | 0.922 | n.s. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules 2019, 24, 2810. https://doi.org/10.3390/molecules24152810
Papalia T, Sidari R, Panuccio MR. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules. 2019; 24(15):2810. https://doi.org/10.3390/molecules24152810
Chicago/Turabian StylePapalia, Teresa, Rossana Sidari, and Maria Rosaria Panuccio. 2019. "Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass" Molecules 24, no. 15: 2810. https://doi.org/10.3390/molecules24152810
APA StylePapalia, T., Sidari, R., & Panuccio, M. R. (2019). Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules, 24(15), 2810. https://doi.org/10.3390/molecules24152810






