Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia obtusifolia)
Abstract
:1. Introduction
2. Results and Discussions
2.1. Optimization of Microwave-Assisted Extraction of Polysaccharides from Cassia Seed
2.2. Comparison of Physicochemical Characteristics of CSPs from Cassia Seed Extracted by HWE (CSP-W) and MAE (CSP-M)
2.2.1. Chemical Compositions of CSP-W and CSP-M
2.2.2. Molecular Weights, Intrinsic Viscosities, and Constituent Monosaccharides of CSP-W and CSP-M
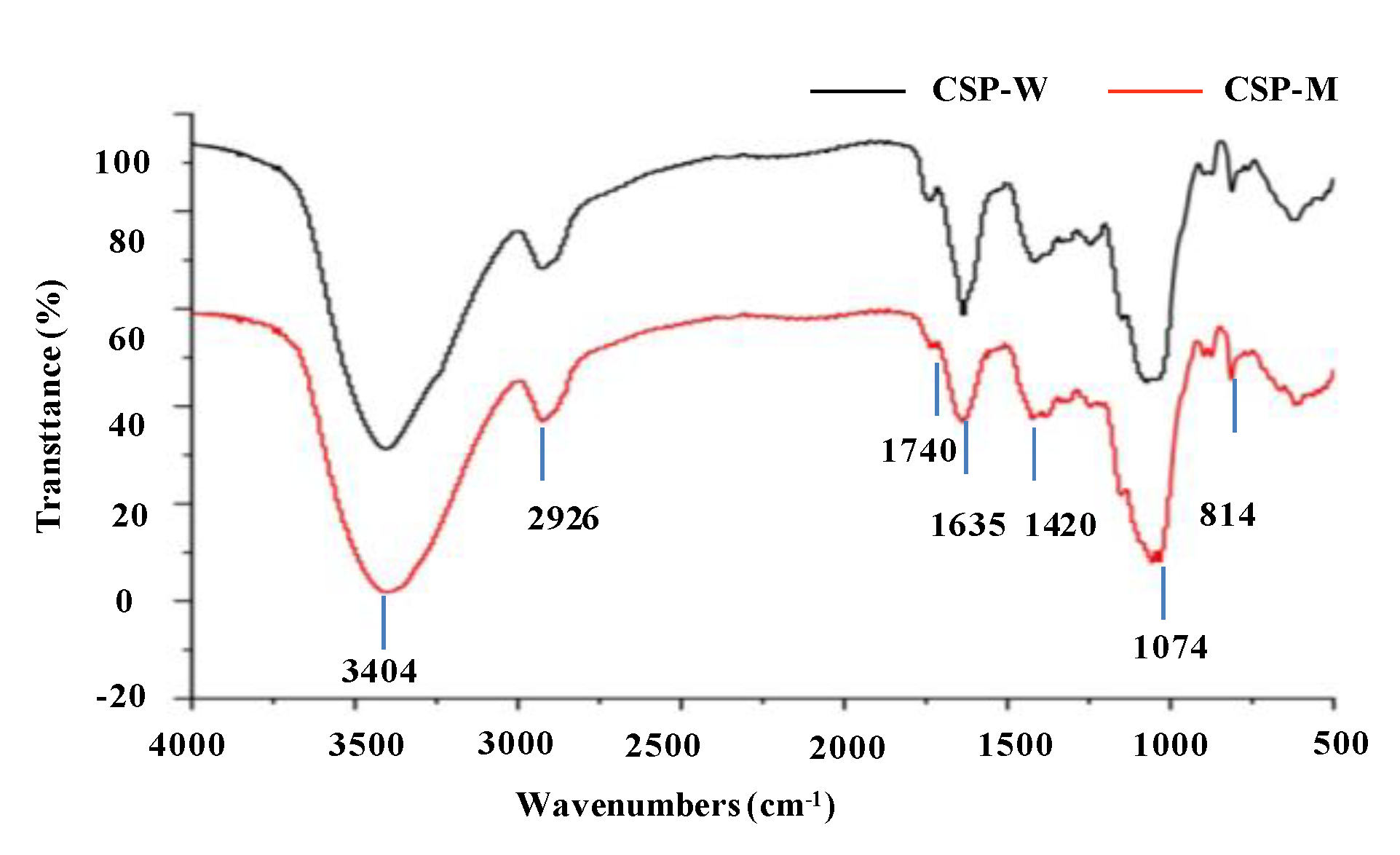
2.2.3. Fourier Transform Infrared Spectra and Degree of Esterification of CSP-W and CSP-M
2.3. Comparison of Antioxidant Activities of CSPs from Cassia Seed Extracted by HWE and MAE
3. Material and Methods
3.1. Samples and Chemicals
3.2. Extraction of Polysaccharides from Cassia Seed
3.2.1. Hot Water Extraction of CSPs
3.2.2. Microwave-Assisted Extraction of CSPs
3.3. Characterization of Polysaccharides from Cassia Seed Extracted by HWE and MAE
3.3.1. Chemical Composition Analysis
3.3.2. Determination of Molecular Weights of CSP-W and CSP-M
3.3.3. Determination of Intrinsic Viscosities of CSP-W and CSP-M
3.3.4. Determination of Constituent Monosaccharides of CSP-W and CSP-M
3.3.5. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
3.4. Evaluation of Antioxidant Activities of Polysaccharides from Cassia Seed Extracted by HWE and MAE
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qian, Z.J.; Ryu, B.; Kim, M.M.; Kim, S.K. Free radical and reactive oxygen species scavenging activities of the extracts from seahorse, Hippocampus kuda Bleeler. Biotechnol. Bioprocess Eng. 2008, 13, 705–715. [Google Scholar] [CrossRef]
- Liu, C.J.; Liu, Q.; Sun, J.D.; Jiang, B.; Yan, J.F. Extraction of water-soluble polysaccharide and the antioxidant activity from Semen Cassiae. J. Food Drug Anal. 2014, 22, 492–499. [Google Scholar] [CrossRef]
- Liang, X.X.; Gao, Y.Y.; Fei, W.B.; Zou, Y.F.; He, M.; Yin, L.Z.; Yuan, Z.X.; Yin, Z.Q.; Zhang, W. Chemical characterization and antioxidant activities of polysaccharides isolated from the stems of Parthenocissus tricuspidata. Int. J. Biol. Macromol. 2018, 119, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.G.; Jia, S.R.; Wu, Y.K.; Yan, R.R.; Lin, Y.H.; Zhao, D.X.; Han, P.P. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr. Polym. 2018, 198, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Huang, G.L. Preparation and antioxidant activities of cuaurbit polysaccharide. Int. J. Biol. Macromol. 2018, 117, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Capek, P.; Machová, E.; Turjan, J. Scavenging and antioxidant activities of immunomodulating polysaccharides isolated from Salvia officinalis L. Int. J. Biol. Macromol. 2009, 44, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Zhang, T.; Jiang, B.; Mu, W.M.; Miao, M. Characterization and antioxidant activity of Ginkgo biloba exocarp polysaccharides. Carbohydr. Polym. 2012, 87, 40–45. [Google Scholar] [CrossRef]
- Hu, H.B.; Liang, H.P.; Li, H.M.; Yuan, R.N.; Sun, J.; Zhang, L.L.; Han, M.H.; Wu, Y. Isolation, purification, characterization and antioxidant activity of polysaccharides from the stem barks of Acanthopanax leucorrhizus. Carbohydr. Polym. 2018, 196, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Subramonian, W.; Wu, T.Y.; Chai, S.P. A comprehensive study on coagulant performance and floc characterization of natural Cassia obtusifolia seed gum in treatment of raw pulp and paper mill effluent. Ind. Crops Prod. 2014, 61, 317–324. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Barros, W.; Santos, G.R.C.; Correia, M.T.S.; Mourao, P.A.S.; Teixeira, J.A.; Carneiro da Cunha, M.G. Characterization and rheological study of the galactomannan extracted from seeds of Cassia grandis. Carbohydr. Polym. 2014, 104, 127–134. [Google Scholar] [CrossRef]
- Feng, L.; Yin, J.Y.; Nie, S.P.; Wan, Y.Q.; Xie, M.Y. Fractionation, physicochemical property and immunological activity of polysaccharides from Cassia obtusifolia. Int. J. Biol. Macromol. 2016, 91, 946–953. [Google Scholar] [CrossRef]
- Sharmila, G.; Nikitha, V.S.; Ilaiyarasi, S.; Dhivya, K.; Rajasekar, V.; Kumar, N.M.; Muthukumaran, K.; Muthukumaran, C. Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2016, 84, 13–21. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Yu, B. Total synthesis of the antiallergic naphtho-α-pyrone tetraglucoside, cassiaside C2, isolated from cassia seeds. J. Org. Chem. 2003, 68, 6309–6313. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Chan, C.L.; Gan, R.Y.; Corke, H. The phenolic composition and antioxidant capacity of soluble and bound extracts in selected dietary spices and medicinal herbs. Int. J. Food Sci. Technol. 2016, 51, 565–573. [Google Scholar] [CrossRef]
- Huang, Y.L.; Chow, C.J.; Tsai, Y.H. Composition, characteristics, and in-vitro physiological effects of the water-soluble polysaccharides from Cassia seed. Food Chem. 2012, 134, 1967–1972. [Google Scholar] [CrossRef]
- Feng, L.; Yin, J.Y.; Nie, S.P.; Wan, Y.Q.; Xie, M.Y. Structure and conformation characterization of galactomannan from seeds of Cassia obtusifolia. Food Hydrocoll. 2018, 76, 67–77. [Google Scholar] [CrossRef]
- Feng, L.; Yin, J.Y.; Nie, S.P.; Wan, Y.Q.; Xie, M.Y. Enzymatic purification and structure characterization of glucuronoxylan from water extract of Cassia obtusifolia seeds. Int. J. Biol. Macromol. 2018, 107, 1438–1446. [Google Scholar] [CrossRef]
- Cong, Q.F.; Shang, M.S.; Dong, Q.; Liao, W.F.; Xiao, F.; Ding, K. Structure and activities of a novel heteroxylan from Cassia obtusifolia seeds and its sulfated derivative. Carbohydr. Res. 2014, 393, 43–50. [Google Scholar] [CrossRef]
- Dong, H.M.; Zhang, Q.; Li, Y.; Li, L.; Lan, W.J.; He, J.L.; Li, H.Y.; Xiong, Y.B.; Qin, W. Extraction, characterization and antioxidant activities of polysaccharides of Chuanminshen violaceum. Int. J. Biol. Macromol. 2016, 86, 224–232. [Google Scholar] [CrossRef]
- Han, Q.H.; Liu, W.; Li, H.Y.; He, J.L.; Guo, H.; Lin, S.; Zhao, L.; Chen, H.; Liu, Y.W.; Wu, D.T.; et al. Extraction optimization, physicochemical characteristics, and antioxidant activities of polysaccharides from kiwifruit (Actinidia chinensis Planch.). Molecules 2019, 24, 461. [Google Scholar] [CrossRef]
- Chen, G.J.; Chen, K.W.; Zhang, R.F.; Chen, X.L.; Hu, P.; Kan, J.Q. Polysaccharides from bamboo shoots processing by-products: New insight into extraction and characterization. Food Chem. 2018, 245, 1113–1123. [Google Scholar] [CrossRef]
- Ren, B.B.; Chen, C.; Li, C.; Fu, X.; You, L.J.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xue, Y.T. Optimization of microwave assisted extraction, chemical characterization and antitumor activities of polysaccharides from porphyra haitanensis. Carbohydr. Polym. 2019, 206, 179–186. [Google Scholar] [CrossRef]
- Sun, H.Y.; Li, C.Y.; Ni, Y.J.; Yao, L.P.; Jiang, H.W.; Ren, X.T.; Fu, Y.J.; Zhao, C.J. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym. 2019, 206, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Hosseini, S.S.; Hojjati, M. Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem. 2019, 271, 663–672. [Google Scholar] [CrossRef]
- Silva, A.D.E.; de Magalhães, W.T.; Moreira, L.M.; Rocha, M.V.P.; Bastos, A.K.P. Microwave-assisted extraction of polysaccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res. 2018, 35, 178–184. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.L.; Zou, P.; Zhang, C.S.; Li, Y.Q. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Dong, H.M.; Lin, S.; Zhang, Q.; Chen, H.; Lan, W.J.; Li, H.Y.; He, J.L.; Qin, W. Effect of extraction methods on the properties and antioxidant activities of Chuanminshen violaceum polysaccharides. Int. J. Biol. Macromol. 2016, 93, 179–185. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.H.; Liu, H.; Wu, C.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Extraction optimization and effects of extraction methods on the chemical structures and antioxidant activities of polysaccharides from snow chrysanthemum (Coreopsis Tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Tang, X.Y.; Fan, H.J.; Xie, X.J.; Wan, Q.; Wu, X.H.; Tang, J.Z. Extraction and characterization of polysaccharides from Semen Cassiae by microwave-assisted aqueous two-phase extraction coupled with spectroscopy and HPLC. Carbohydr. Polym. 2016, 144, 263–270. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Liu, R.H. Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: Characterization and hypoglycemic activity. Ind. Crop. Prod. 2017, 100, 1–11. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zhang, M.P.; Zhou, H.L. Microwave-assisted extraction, purification, partial characterization, and bioactivity of polysaccharides from Panax ginseng. Molecules 2019, 24, 1605. [Google Scholar] [CrossRef]
- Wang, Y.G.; Xu, Y.; Ma, X.Q.; Liu, X.F.; Yang, M.J.; Fan, W.G.; Ren, H.W.; Efehi, N.; Wang, X.L.; Zhu, X.Q. Extraction, purification, characterization and antioxidant activities of polysaccharides from Zizyphus jujuba cv. Linzexiaozao. Int. J. Biol. Macromol. 2018, 118, 2138–2148. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Wang, P.H.; Zhou, H.L.; Li, Y.P. Extraction, characterization and in vitro antioxidant activity of polysaccharides from Carex meyeriana Kunth using different methods. Int. J. Biol. Macromol. 2018, 120, 2155–2164. [Google Scholar] [CrossRef]
- Wu, D.T.; Guo, H.; Lin, S.; Lam, S.C.; Zhao, L.; Lin, D.R.; Qin, W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food Sci. Technol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- He, L.; Yan, X.T.; Liang, J.; Li, S.J.; He, H.R.; Xiong, Q.P.; Lai, X.P.; Hou, S.Z.; Huang, S. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydr. Polym. 2018, 198, 101–108. [Google Scholar] [CrossRef]
- Deore, U.V.; Mahajan, H.S. Isolation and characterization of natural polysaccharide from Cassia Obtustifolia seed mucilage as film forming material for drug delivery. Int. J. Biol. Macromol. 2018, 115, 1071–1078. [Google Scholar] [CrossRef]
- Shang, M.S.; Zhang, X.M.; Dong, Q.; Yao, J.; Liu, Q.; Ding, K. Isolation and structural characterization of the water-extractable polysaccharides from Cassia obtusifolia seeds. Carbohydr. Polym. 2012, 90, 827–832. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.P.; Du, C.Y.; Mou, H.J.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
- Liang, X.X.; Gao, Y.Y.; Pan, Y.; Zou, Y.F.; He, M.; He, C.L.; Li, L.X.; Yin, Z.Q.; Lv, C. Purification, chemical characterization and antioxidant activities of polysaccharides isolated from Mycena dendrobii. Carbohydr. Polym. 2019, 203, 45–51. [Google Scholar] [CrossRef]
- Gu, D.; Huang, L.L.; Chen, X.; Wu, Q.H.; Ding, K. Structural characterization of a galactan from Ophiopogon japonicus and anti-pancreatic cancer activity of its acetylated derivative. Int. J. Biol. Macromol. 2018, 113, 907–915. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, Q.; Lin, S.; Liu, W.; Du, G.; Zhao, L.; Zhang, Q.; Lin, D.R.; Liu, Y.T.; Qin, W.; et al. Physicochemical characteristics and biological activities of polysaccharides from the leaves of different loquat (Eriobotrya japonica) cultivars. Int. J. Biol. Macromol. 2019, 135, 274–281. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Wai, W.W.; Alkarkhi, A.F.M.; Easa, A.M. Effect of extraction conditions on yield and degree of esterification of durian rind pectin: An experimental design. Food Bioprod. Process. 2010, 88, 209–214. [Google Scholar] [CrossRef]
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Yellow lupin (Lupinus luteus L.) polysaccharides: Antioxidant, immunomodulatory and prebiotic activities and their structural characterisation. Food Chem. 2018, 267, 319–328. [Google Scholar] [CrossRef]
- Ji, Y.H.; Liao, A.M.; Huang, J.H.; Thakur, K.; Li, X.L.; Wei, Z.J. Physicochemical and antioxidant potential of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 131, 453–460. [Google Scholar] [CrossRef]
- Li, L.; Thakur, K.; Liao, B.Y.; Zhang, J.G.; Wei, Z.J. Antioxidant and antimicrobial potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 114, 317–323. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Lv, G.Y.; He, W.Q.; Shi, L.G.; Pan, H.J.; Fan, L.F. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef]
- Chai, Z.; Huang, W.Y.; Zhao, X.; Wu, H.; Zeng, X.X.; Li, C.Y. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of polysaccharide from Cynanchum auriculatum Royle ex Wight. Int. J. Biol. Macromol. 2018, 119, 1068–1076. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Lu, M.; Lu, M.Y.; Gong, J.D.B.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.T. Correlations of molecular weights of β-glucans from Qingke (Tibetan hulless barley) to Their Multiple Bioactivities. Molecules 2018, 23, 1710. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filisetti Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.T. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Dong, Y.H.; Qi, Y.R.; Liu, M.; Song, X.L.; Zhang, C.; Jiao, X.; Wang, W.S.; Zhang, J.J.; Jia, L. Antioxidant, anti-hyperlipidemia and hepatic protection of enzyme-assisted Morehella esculenta polysaccharide. Int. J. Biol. Macromol. 2018, 120, 1490–1499. [Google Scholar] [CrossRef]




| Variable | Levels of Independent Factors | Extraction Yields % | ||
|---|---|---|---|---|
| X1 (W) | X2 (mL/g) | X3 (min) | ||
| 1 | −1 (320) | 0 (50) | −1 (4) | 6.08 |
| 2 | 0 (400) | 0 (50) | 0 (6) | 7.98 |
| 3 | 0 (400) | 0 (50) | 0 (6) | 7.89 |
| 4 | 1 (480) | 0 (50) | 1 (8) | 7.14 |
| 5 | 0 (400) | 0 (50) | 0 (6) | 8.01 |
| 6 | 1 (480) | 0 (50) | − | 6.83 |
| 7 | 0 (400) | 1 (60) | 1 (8) | 7.34 |
| 8 | −1 (320) | 0 (50) | 1 (8) | 7.01 |
| 9 | −1 (320) | −1 (40) | 0 (6) | 6.28 |
| 10 | 1 (480) | 1 (60) | 0 (6) | 7.12 |
| 11 | 1 (480) | −1 (40) | 0 (6) | 6.72 |
| 12 | 0 (400) | 0 (50) | 0 (6) | 7.96 |
| 13 | −1 (320) | 1 (60) | 0 (6) | 6.37 |
| 14 | 0 (400) | 0 (50) | 0 (6) | 8.04 |
| 15 | 0 (400) | −1 (40) | −1 (4) | 6.38 |
| 16 | 0 (400) | 1 (60) | −1 (4) | 6.19 |
| 17 | 0 (400) | −1 (40) | 1 (8) | 6.60 |
| Source a | Microwave-Assisted Extraction | ||||
|---|---|---|---|---|---|
| Sum of Square | df b | Mean Square | F-Value | p-Value c | |
| Model | 7.90 | 9 | 0.88 | 227.16 | <0.0001 ** |
| X1 | 0.54 | 1 | 0.54 | 139.36 | <0.0001 ** |
| X2 | 0.14 | 1 | 0.14 | 35.42 | 0.0006 ** |
| X3 | 0.85 | 1 | 0.85 | 219.73 | <0.0001 ** |
| X1X2 | 0.023 | 1 | 0.023 | 6.02 | 0.0439 * |
| X1X3 | 0.092 | 1 | 0.092 | 23.88 | 0.0018 ** |
| X2X3 | 0.22 | 1 | 0.22 | 56.26 | 0.0001 ** |
| X12 | 1.55 | 1 | 1.55 | 401.81 | <0.0001 ** |
| X22 | 2.33 | 1 | 2.33 | 603.76 | <0.0001 ** |
| X32 | 1.53 | 1 | 1.53 | 395.76 | <0.0001 ** |
| Residual Error | 0.027 | 7 | 3.864 × 10−3 | ||
| Lack of Fit | 0.015 | 3 | 5.009 × 10−3 | 1.67 | 0.3099 |
| Pure Error | 0.012 | 4 | 3.006 × 10−3 | ||
| Correlation Total | 7.93 | 16 | |||
| Chemical Composition | Samples | |
|---|---|---|
| CSP-W | CSP-M | |
| Extraction Yields (%) | 8.17 ± 0.33 a | 8.02 ±0.19 a |
| Extraction Time (min) | 240 | 7 |
| Extraction Temperature (°C) | 90 | 85 |
| Total Polysaccharides (%) | 80.76 ± 1.19 b | 85.38 ± 1.04 a |
| Total Uronic Acids (%) | 20.14 ± 0.59 a | 18.44 ± 0.67 b |
| Degree of Esterification (%) | 11.88± 0.67 a | 4.70 ± 0.25 b |
| Proteins (%) | 4.80 ± 0.03 b | 5.50 ± 0.12 a |
| Samples | ||
| CSP-W | CSP-M | |
| Mw × 104 (Da, Error) | ||
| Fraction 1 | 133.7 (±1.55%) a | 109.1 (±1.09%) b |
| Fraction 2 | 9.838 (±1.88%) b | 14.760 (±2.33%) a |
| Fraction 3 | 2.514 (±3.66%) b | 3.615 (±4.16%) a |
| Mw/Mn | ||
| Fraction 1 | 1.562 | 1.327 |
| Fraction 2 | 1.423 | 1.234 |
| Fraction 3 | 1.255 | 1.170 |
| [η] (dL/g) | 2.81 ± 0.05 a | 2.70 ± 0.04 b |
| Monosaccharide Compositions (Molar Ratio) | ||
| Mannose | 2.28 | 2.88 |
| Rhamnose | 0.07 | 0.05 |
| Glucuronic Acid | 0.12 | 0.06 |
| Galacturonic Acid | 0.52 | 0.14 |
| Glucose | 0.17 | 0.20 |
| Galactose | 1.00 | 1.00 |
| Xylose | 0.79 | 0.72 |
| Arabinose | 0.29 | 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.-T.; Liu, W.; Han, Q.-H.; Wang, P.; Xiang, X.-R.; Ding, Y.; Zhao, L.; Zhang, Q.; Li, S.-Q.; Qin, W. Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia obtusifolia). Molecules 2019, 24, 2817. https://doi.org/10.3390/molecules24152817
Wu D-T, Liu W, Han Q-H, Wang P, Xiang X-R, Ding Y, Zhao L, Zhang Q, Li S-Q, Qin W. Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia obtusifolia). Molecules. 2019; 24(15):2817. https://doi.org/10.3390/molecules24152817
Chicago/Turabian StyleWu, Ding-Tao, Wen Liu, Qiao-Hong Han, Ping Wang, Xian-Rong Xiang, Ye Ding, Li Zhao, Qing Zhang, Su-Qing Li, and Wen Qin. 2019. "Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia obtusifolia)" Molecules 24, no. 15: 2817. https://doi.org/10.3390/molecules24152817





