Determination of 107 Pesticide Residues in Wolfberry with Acetate-buffered Salt Extraction and Sin-QuEChERS Nano Column Purification Coupled with Ultra Performance Liquid Chromatography Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results
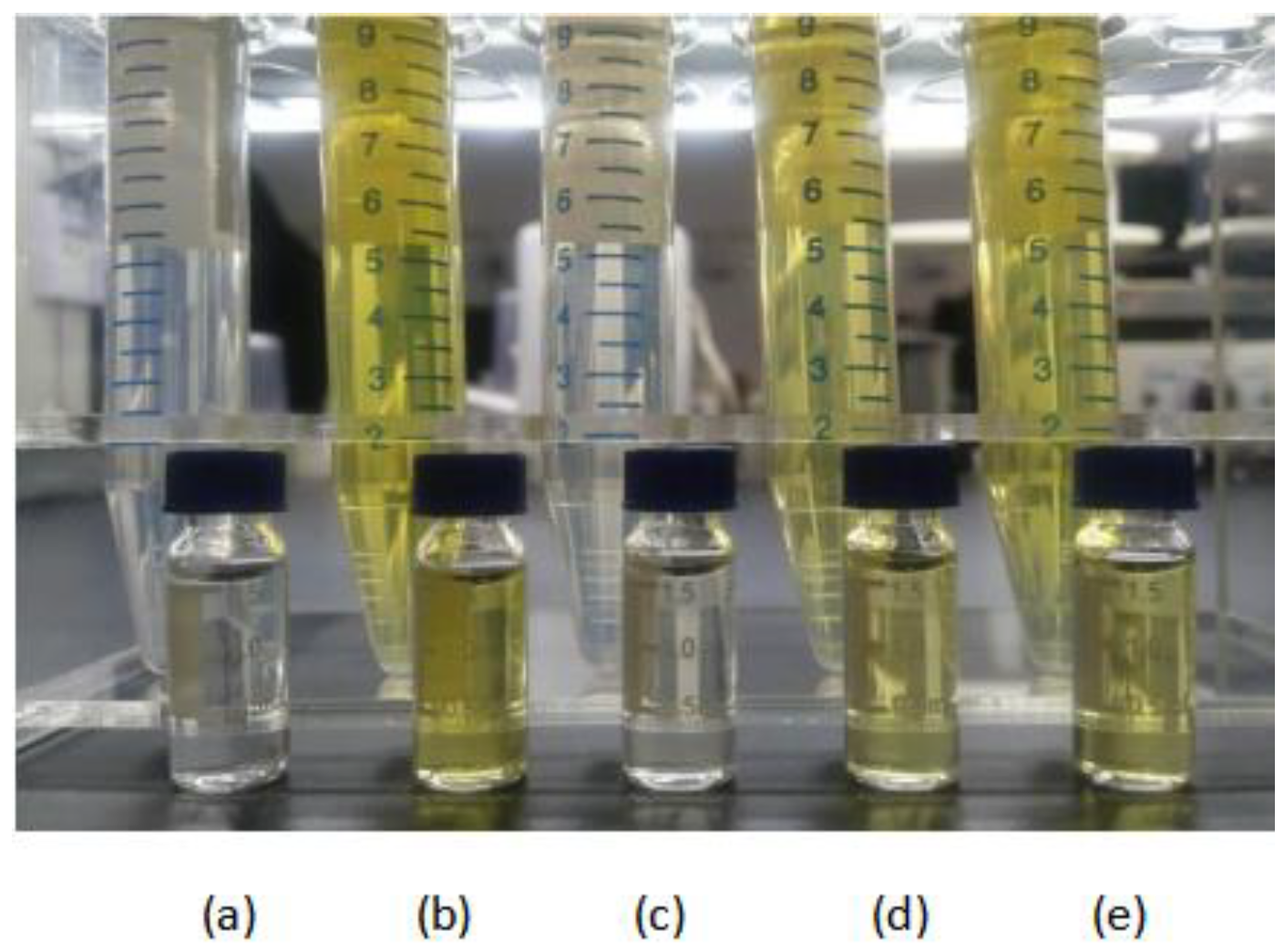
2.1. Optimization and Comparison of the Extraction Procedure
2.2. Optimization and Comparison of the Clean-up Procedure
2.3. Method Validation
2.3.1. Linearity, LOD and LOQ
2.3.2. Precision and Stability
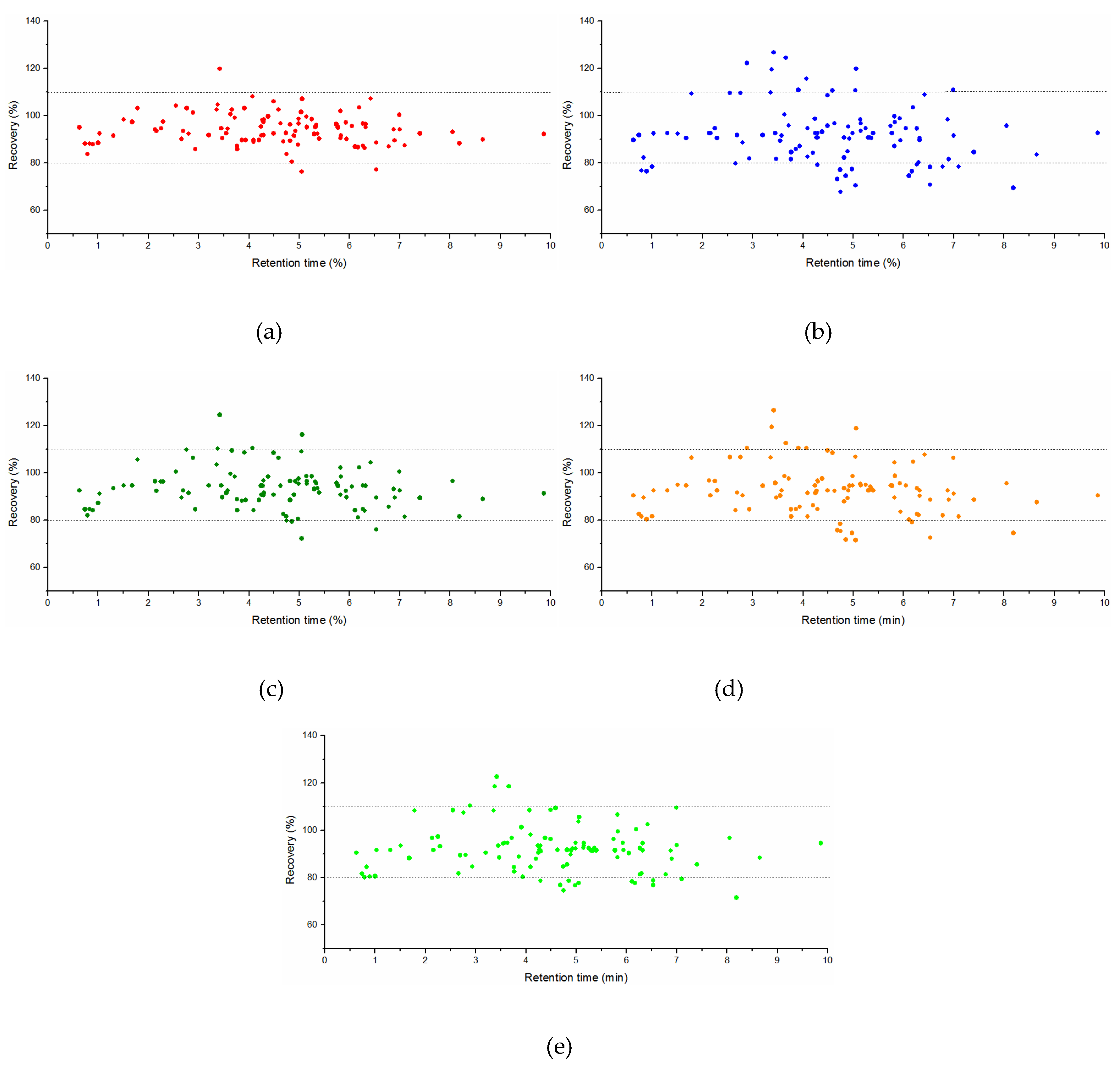
2.3.3. Accuracy
2.4. Matrix Effects
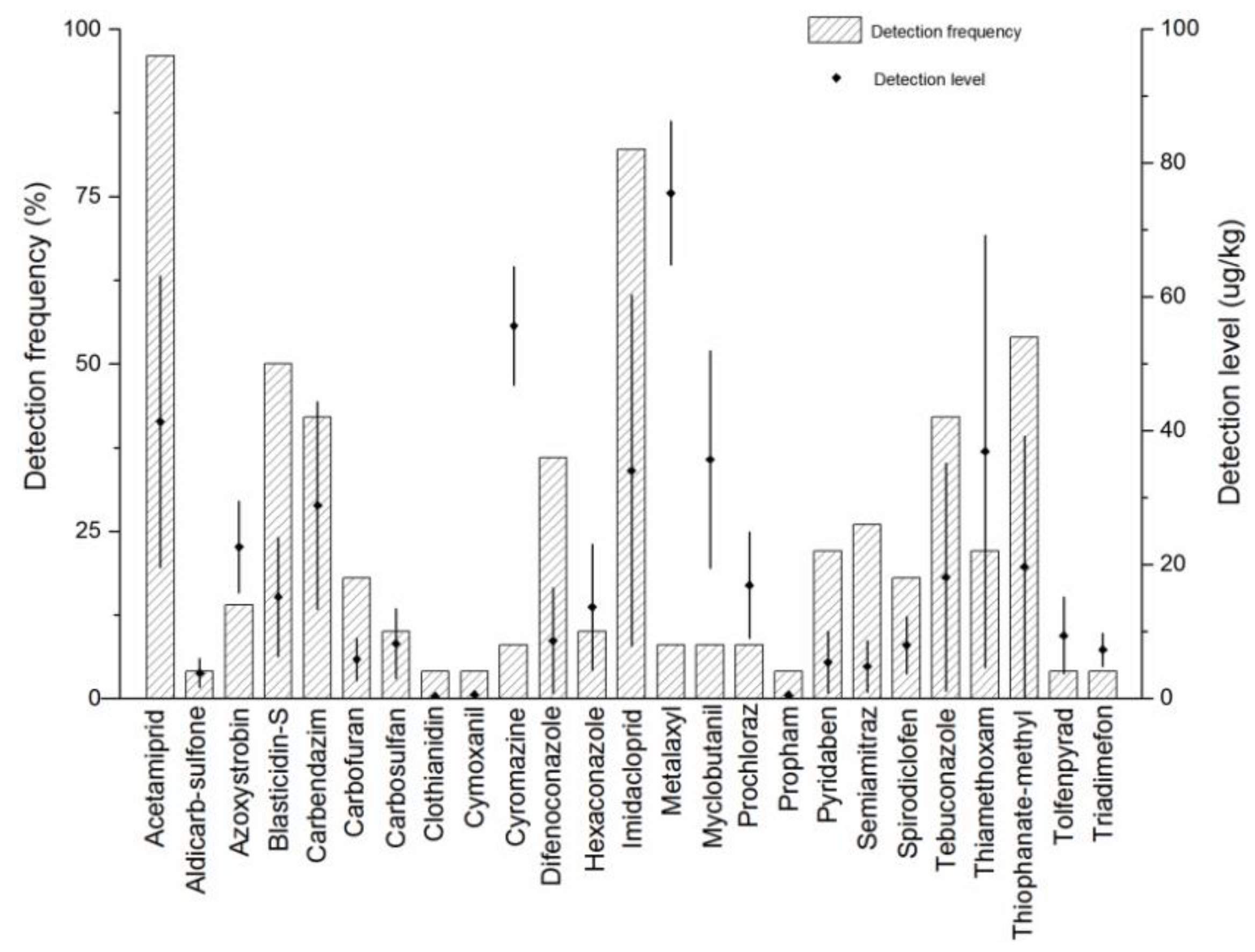
2.5. Real Samples Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrument
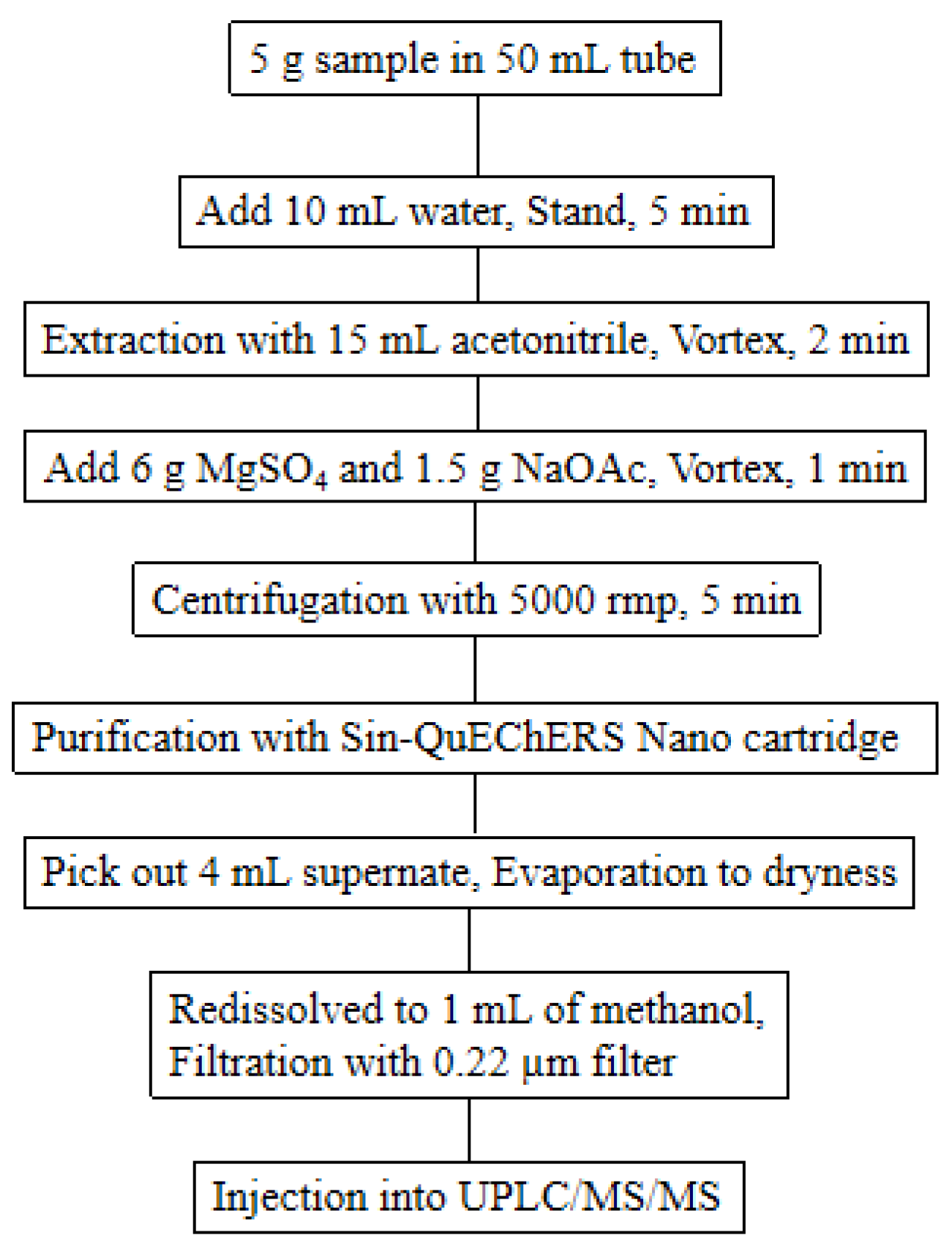
3.3. Sample Preparation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.F.; Chen, J.; Yang, J.; Shi, Y.P. UPLC-MS/MS analysis for antioxidant components of Lycii Fructus based on spectrum-effect relationship. Talanta 2018, 180, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Li, H.X.; Xi, W.P.; An, W.; Niu, L.L.; Cao, Y.L.; Wang, H.F.; Wang, Y.J.; Yin, Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem. 2015, 173, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Huang, Q.S.; Zhao, K.Z.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic review of chemical constituents in the genus Lycium (Solanaceae). Molecules 2017, 22, 911. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.P.; Dang, T.T.; Deng, Y.N.; Han, J.L.; Zou, Z.H.; Jing, S.; Zhang, Y.; Lin, Q.; Huang, L.J.; Wang, Z.F. Physicochemical properties and biologicai activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar]
- Wang, W.J.; Zhang, T. Integration of traditional Chinese medicine and Western medicine in the era of precision medicine. J. Integr. Med. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Wu, D.T.; Guo, H.; Lin, S.; Lam, S.C.; Zhao, L.; Lin, D.R.; Qin, W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food. Sci. Technol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- Xiao, J.J.; Xu, X.; Wang, F.; Ma, J.J.; Liao, M.; Shi, Y.H.; Fang, Q.K.; Cao, H.Q. Analysis of exposure to pesticide residues from Traditional Chinese Medicine. J. Hazard. Mater. 2019, 365, 857–867. [Google Scholar] [CrossRef]
- Sun, Y.; Rukeya, J.; Tao, W.; Sun, P.; Ye, X. Bioactive compounds and antioxidant activity of wolfberry infusion. Sci. Rep. 2017, 7, 40605. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.L.; Dong, F.S.; Wu, X.H.; Xu, J.; Liu, X.G.; Zheng, Y.Q. Progress of the discovery, application, and control technologies of chemical pesticides in China. J. Integr. Agric. 2019, 18, 840–853. [Google Scholar] [CrossRef] [Green Version]
- Zhan, X.P.; Ma, L.; Huang, L.Q.; Chen, J.B.; Zhao, L. The optimization and establishment of QuEChERS-UPLC-MS/MS method for simultaneously detecting various kinds of pesticides residues in fruits and vegetables. J. Chromatogr. B 2017, 1060, 281–290. [Google Scholar]
- Diop, A.; Diop, Y.M.; Thiare, D.D.; Cazier, F.; Sarr, S.O.; Kasprowiak, A.; Landy, D.; Delattre, F. Monitoring survey of the use patterns and pesticide residues on vegetables in the Niayes zone, Senegal. Chemosphere 2016, 144, 1715–1721. [Google Scholar] [CrossRef]
- Chen, X.X.; Li, X.; Pang, K.J.; Fan, X.Q.; Ma, Y.C.; Hu, J.Y. Dissipation behavior and residue distribution of fluazaindolizine and its seven metabolites in tomato ecosystem based on SAX SPE procedure using HPLC-QqQ-MS/MS technique. J. Hazard. Mater. 2018, 342, 698–704. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Khoshmaram, L. Air-assisted liquid-liquid microextraction-gas chromatography-flame ionisation detection: A fast and simple method for the assessment of triazole pesticides residues in surface water, cucumber, tomato and grape juices samples. Food Chem. 2013, 141, 1881–1887. [Google Scholar] [CrossRef]
- Taha, S.M.; Gadalla, S.A. Development of an efficient method for multi residue analysis of 160 pesticides in herbal plant by ethyl acetate hexane mixture with direct injection to GC-MS/MS. Talanta 2017, 174, 767–779. [Google Scholar] [CrossRef]
- Ji, W.H.; Sun, R.H.; Duan, W.J.; Wang, X.; Wang, T.; Mu, Y.; Guo, L.P. Selective solid phase extraction of chloroacetamide herbicides from environmental water samples by amphiphilic magnetic molecularly imprinted polymers. Talanta 2017, 170, 111–118. [Google Scholar] [CrossRef]
- Bandforuzi, S.R.; Hadjmohammadi, M.R. Modified magnetic chitosan nanoparticles based on mixed hemimicelle of sodium dodecyl sulfate for enhanced removal and trace determination of three organophosphorus pesticides from natural waters. Anal. Chim. Acta 2019, 1078, 90–100. [Google Scholar] [CrossRef]
- Gil García, M.D.; Uclés Duque, S.; Lozano Fernández, A.B.; Sosa, A.; Fernández-Alba, A.R. Multiresidue method for trace pesticide analysis in honeybee wax comb by GC-QqQ-MS. Talanta 2017, 163, 54–64. [Google Scholar] [CrossRef]
- Wang, S.C.; Qi, P.P.; Di, S.S.; Wang, J.; Wu, S.G.; Wang, X.Y.; Wang, Z.W.; Wang, Q.; Wang, X.Q.; Zhao, C.S.; et al. Significant role of supercritical fluid chromatography-mass spectrometry in improving the matrix effect and analytical efficiency during multi-pesticides residue analysis of complex chrysanthemum samples. Anal. Chim. Acta 2019, 1074, 108–116. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhao, K.X.; Ge, B.K.; Chen, Q.Y. Simultaneous determination of 44 pesticides in tobacco by UPLC/MS/MS and a modified QuEChERS procedure. J. AOAC Int. 2013, 96, 422–431. [Google Scholar] [CrossRef]
- Madej, K.; Kalenik, T.K.; Piekoszewski, W. Sample preparation and determination of pesticides in fat-containing foods. Food Chem. 2018, 269, 527–541. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends. Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Bresin, B.; Piol, M.; Fabbro, D.; Mancini, M.A.; Casetta, B.; Bianco, C.D. Analysis of organo-chlorine pesticides residue in raw coffee with a modified “quick easy cheap effective rugged and safe” extraction/clean up procedure for reducing the impact of caffeine on the gas chromatography-mass spectrometry measurement. J. Chromatogr. A 2015, 1376, 167–171. [Google Scholar] [CrossRef]
- Jian, W.; Wendy, C. UHPLC/ESI-MS/MS Determination of 187 Pesticides in Wine. J. AOAC Int. 2016, 99, 539–557. [Google Scholar]
- Guo, J.G.; Tong, M.M.; Tang, J.; Bian, H.Z.; Wan, X.C.; He, L.L.; Hou, R.Y. Analysis of multiple pesticide residues in polyphenol-rich agricultural products by UPLC-MS/MS using a modified QuEChERS extraction and dilution method. Food Chem. 2019, 274, 452–459. [Google Scholar] [CrossRef]
- Tighrine, A.; Amir, Y.; Alfaro, P.; Mamou, M.; Nerín, C. Simultaneous extraction and analysis of preservatives and artificial sweeteners in juices by salting out liquid-liquid extraction method prior to ultra-high performance liquid chromatography. Food Chem. 2019, 277, 586–594. [Google Scholar] [CrossRef]
- Castro, G.; Rodríguez, I.; Ramil, M.; Cela, R. Selective determination of sartan drugs in environmental water samples by mixed-mode solid-phase extraction and liquid chromatography tandem mass spectrometry. Chemosphere 2019, 224, 562–571. [Google Scholar] [CrossRef]
- Bartosiak, M.; Jankowski, K.; Giersz, J. Determination of cobalt species in nutritional supplements using ICP-OES after microwave-assisted extraction and nutritional supplements using solid-phase extraction. J. Pharm. Biomed. 2018, 155, 135–140. [Google Scholar] [CrossRef]
- Di, X.; Wang, X.; Liu, Y.P.; Guo, X.J.; Di, X. Microwave assisted extraction in combination with solid phase purification and switchable hydrophilicity solvent-based homogeneous liquid-liquid microextraction for the determination of sulfonamides in chicken meat. J. Chromatogr. B 2019, 1118–1119, 109–115. [Google Scholar] [CrossRef]
- Yen, H.W.; Yang, S.C.; Chen, C.H.; Chang, J.S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Shehzad, A. Solvent and temperature effects of accelerated solvent extraction (ASE) with Ultra-high pressure liquid chromatography (UHPLC-PDA) technique for determination of Piperine and its ICP-MS analysis. Ind. Crops Prod. 2019, 136, 37–49. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Zhang, Y.J.; Zhang, W.; Boyd, S.A.; Li, H. Comparison of accelerated solvent extraction and quick, easy, cheap, effective, rugged and safe method for extraction and determination of pharmaceuticals in vegetables. J. Chromatogr. A 2015, 1404, 1–9. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kundu, A.; Sharma, K.; Paul, R.K. Optimal extraction and fingerprinting of carotenoids by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2015, 177, 369–375. [Google Scholar] [CrossRef]
- Liang, L.; Wang, X.H.; Sun, Y.; Ma, P.Y.; Li, X.P.; Piao, H.L.; Jiang, Y.X.; Song, D.Q. Magnetic solid-phase extraction of triazine herbicides from rice using metal-organic framework MIL-101(Cr) functionalized magnetic particles. Talanta 2018, 179, 512–519. [Google Scholar] [CrossRef]
- Ma, J.P.; Wu, G.G.; Li, S.; Tan, W.Q.; Wang, X.Y.; Li, J.H.; Chen, L.X. Magnetic solid-phase extraction of heterocyclic pesticides in environmental water samples using metal-organic frameworks coupled to high performance liquid chromatography determination. J. Chromatogr. A 2018, 1553, 57–66. [Google Scholar] [CrossRef]
- Lian, W.L.; Ren, F.L.; Tang, L.Y.; Dong, D.Z. Analysis of polycyclic aromatic hydrocarbons in cigarette samples using gel permeation chromatography clean-up by gas chromatography-tandem mass spectrometry. Microchem. J. 2016, 129, 194–199. [Google Scholar] [CrossRef]
- Qian, M.R.; Zhang, H.; Wu, L.Q.; Jin, N.; Wang, J.M.; Jiang, K.Z. Simultaneous determination of zearalenone and its derivatives in edible vegetable oil by gel permeation chromatography and gas chromatography-triple quadrupole mass spectrometry. Food Chem. 2015, 166, 23–28. [Google Scholar] [CrossRef]
- Zheng, G.C.; Han, C.; Liu, Y.; Wang, J.; Zhu, M.W.; Wang, C.J.; Shen, Y. Multiresidue analysis of 30 organochlorine pesticides in milk and milk powder by gel permeation chromatography-solid phase extraction-gas chromatography-tandem mass spectrometry. J. Dairy Sci. 2014, 97, 6016–6026. [Google Scholar] [CrossRef] [Green Version]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Computational-Based Study of QuEChERS Extraction of Cyclohexanedione Herbicide Residues in Soil by Chemometric Modeling. Molecules 2018, 23, 2009. [Google Scholar] [CrossRef]
- Lehotay, S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar]
- Foods of Plant Origin—Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE-QuEChERS-Method. Available online: www.cen.eu (accessed on 20 February 2009).
- Chen, L.N.; Song, F.R.; Liu, Z.Q.; Zheng, Z.; Xing, J.P.; Liu, S.Y. Multi-residue method for fast determination of pesticide residues in plants used in traditional chinese medicine by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2012, 1225, 132–140. [Google Scholar] [CrossRef]
- Liu, H.M.; Kong, W.J.; Qi, Y.; Gong, B.; Miao, Q.; Wei, J.H.; Yang, M.H. Streamlined pretreatment and GC-FPD analysis of multi-pesticide residues in perennial Morinda roots: A tropical or subtropical plant. Chemosphere 2014, 95, 33–40. [Google Scholar] [CrossRef]
- Miao, Q.; Kong, W.J.; Yang, S.H.; Yang, M.H. Rapid analysis of multi-pesticide residues in lotus seeds by a modified QuEChERS-based extraction and GC-ECD. Chemosphere 2013, 91, 955–962. [Google Scholar] [CrossRef]
- Han, Y.G.; Zou, N.; Song, L.; Li, Y.J.; Qin, Y.H.; Liu, S.W.; Li, X.S.; Pan, C.P. Simultaneous determination of 70 pesticide residues in leek, leaf lettuce and garland chrysanthemum using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J. Chromatogr. B 2015, 1005, 56–64. [Google Scholar] [CrossRef]
- Fan, S.F.; Zhao, P.Y.; Yu, C.S.; Pan, C.P.; Li, X.S. Simultaneous determination of 36 pesticide residues in spinach and cauliflower by LC-MS/MS using multi-walled carbon nanotubes-based dispersive solid-phase clean-up. Food Addit. Contam. A 2014, 31, 73–82. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, P.; Min, G.; Fang, G.Z. Multi-residue determination of pesticides in water using multi-walled carbon nanotubes solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1165, 166–171. [Google Scholar] [CrossRef]
- Hou, X.; Lei, S.R.; Qin, S.T.; Guo, L.A.; Yi, S.G.; Lin, W. A multi-residue method for the determination of pesticides in tea using multi-walled carbon nanotubes as a dispersive solid phase extraction absorbent. Food Chem. 2014, 153, 121–129. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Xu, X.Y.; Luo, J.W.; Jin, S.Q.; Chen, W.Q.; Lin, Z.; Tian, C.X. Simultaneous determination of 131 pesticides in tea by on-line GPC-GC-MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem. 2019, 276, 202–208. [Google Scholar] [CrossRef]
- Han, Y.T.; Song, L.; Zou, N.; Chen, R.H.; Qin, Y.H.; Pan, C.P. Multi-residue determination of 171 pesticides in cowpea using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J. Chromatogr. B 2016, 1031, 99–108. [Google Scholar] [CrossRef]
- Zhao, P.F.; Wang, Z.K.; Li, K.J.; Guo, X.J.; Zhao, L.S. Multi-residue enantiomeric analysis of 18 chiral pesticides in water, soil and river sediment using magnetic solid-phase extraction based on amino modified multiwalled carbon nanotubes and chiral liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2018, 1568, 8–21. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wang, J.H.; Li, L.; Wang, Y. Determination of 103 Pesticides and Their Main Metabolites in Animal Origin Food by QuEChERS and Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods 2017, 10, 1826–1843. [Google Scholar] [CrossRef]
- Han, Y.T.; Xu, J.; Dong, F.S.; Li, W.M.; Liu, X.G.; Li, Y.B.; Kong, Z.Q.; Zhu, Y.L.; Liu, N.; Zheng, Y.Q. The fate of spirotetramat and its metabolite spirotetramat-enol in apple samples during apple cider processing. Food Control 2013, 34, 283–290. [Google Scholar] [CrossRef]
- Niessen, W.; Manini, P.; Andreoli, R. Matrix effects in quantitative pesticide analysis using liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2006, 25, 881–899. [Google Scholar] [CrossRef]
- Pan, X.L.; Dong, F.S.; Xu, J.; Liu, X.G.; Chen, Z.L.; Liu, N.; Chen, X.X.; Tao, Y.; Zhang, H.J.; Zheng, Y.Q. Simultaneous determination of chlorantraniliprole and cyantraniliprole in fruits, vegetables and cereals using ultra-high-performance liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal. Biolanal. Chem. 2015, 407, 4111–4120. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS (Article). Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| No. | Compound | Elemental composition | Precursor ion | Retention Time (min) | Precursor ions (m/z) | Products (m/z) | Cone voltage (V) | Collision energy (qv) | LOD (µg/kg) | LOQ (µg/kg) | Linear range (µg/kg) | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abamectin | C48H72O14 | [M + NH4]+ | 8.65 | 890.7 | 305.1 | 18 | 22 | 1.48 | 3.94 | 5–1000 | 0.9998 |
| 890.7 | 567.2 | 18 | 10 | |||||||||
| 2 | Acephate | C4H10NO3PS | [M + H]+ | 0.79 | 184.0 | 49.2 | 10 | 16 | 1.17 | 3.91 | 5–1000 | 0.9981 |
| 3 | Acetamiprid | C10H11ClN4 | [M + H]+ | 2.29 | 223.0 | 56.1 | 22 | 10 | 0.46 | 1.54 | 5–1000 | 0.9999 |
| 223.0 | 126.0 | 22 | 12 | |||||||||
| 4 | Acetochlor | C14H20ClNO2 | [M + H]+ | 5.14 | 270.0 | 148.1 | 15 | 20 | 0.27 | 0.90 | 2–1000 | 0.9995 |
| 270.0 | 224.0 | 15 | 10 | |||||||||
| 5 | Aldicarb | C7H14N2O2S | [M + Na]+ | 2.80 | 213.1 | 89.1 | 25 | 16 | 0.45 | 1.50 | 5–1000 | 0.9697 |
| 213.1 | 116.1 | 25 | 11 | |||||||||
| 6 | Aldicarb-Sulfone | C7H14N2O4S | [M + H]+ | 1.30 | 223.0 | 86.0 | 25 | 10 | 0.72 | 2.39 | 5–1000 | 0.9990 |
| 223.0 | 148.0 | 25 | 8 | |||||||||
| 7 | Aldicarb-Sulfoxide | C7H14N2O3S | [M + H]+ | 0.89 | 207.1 | 89.0 | 10 | 14 | 0.68 | 2.27 | 5–1000 | 0.9911 |
| 207.1 | 132.0 | 10 | 5 | |||||||||
| 8 | Ametryn | C9H17N5S | [M + H]+ | 2.89 | 228.1 | 68.1 | 25 | 25 | 0.50 | 1.66 | 5–1000 | 0.9998 |
| 228.1 | 186.0 | 25 | 10 | |||||||||
| 9 | Atrazine | C8H14ClN5 | [M + H]+ | 3.66 | 216.1 | 96.1 | 22 | 18 | 0.90 | 3.00 | 5–1000 | 0.9996 |
| 216.1 | 174.1 | 22 | 14 | |||||||||
| 10 | Azoxystrobin | C22H17N3O5 | [M + H]+ | 4.63 | 404.0 | 329.0 | 25 | 25 | 0.33 | 1.08 | 5–1000 | 0.9995 |
| 404.0 | 372.0 | 25 | 12 | |||||||||
| 11 | Bendiocarb | C11H13NO4 | [M + H]+ | 3.38 | 224.1 | 109.0 | 17 | 18 | 0.24 | 0.79 | 2–1000 | 0.9987 |
| 224.1 | 167.0 | 17 | 8 | |||||||||
| 12 | Blasticidin-S | C17H26N8O5 | [M + H]+ | 2.76 | 423.5 | 189.3 | 18 | 12 | 0.98 | 3.27 | 5–1000 | 0.9994 |
| 423.5 | 210.6 | 18 | 12 | |||||||||
| 13 | Boscalid | C18H12Cl2N2O | [M − H]− | 4.75 | 342.9 | 139.9 | 32 | 20 | 0.60 | 2.01 | 5–1000 | 0.9994 |
| 342.9 | 307.0 | 32 | 20 | |||||||||
| 14 | Bupirimate | C13H24N4O3S | [M + H]+ | 5.06 | 317.0 | 108.0 | 31 | 28 | 0.48 | 1.61 | 5–1000 | 0.9995 |
| 317.0 | 166.0 | 31 | 28 | |||||||||
| 15 | Buprofezin | C16H23N3OS | [M + H]+ | 5.36 | 306.1 | 116.0 | 20 | 10 | 0.82 | 2.74 | 5–1000 | 0.9997 |
| 306.1 | 201.0 | 20 | 10 | |||||||||
| 16 | Butralin | C14H21N3O4 | [M + H]+ | 8.19 | 296.3 | 222.2 | 20 | 20 | 0.21 | 0.70 | 2–500 | 0.9997 |
| 296.3 | 240.3 | 20 | 17 | |||||||||
| 17 | Carbaryl | C12H11NO2 | [M + H]+ | 3.58 | 202.0 | 117.0 | 19 | 28 | 0.68 | 2.26 | 5–1000 | 0.9998 |
| 202.0 | 145.0 | 19 | 22 | |||||||||
| 18 | Carbendazim | C9H9N3O2 | [M + H]+ | 6.32 | 192.0 | 132.0 | 17 | 22 | 1.11 | 3.70 | 5–1000 | 0.9996 |
| 192.0 | 160.0 | 17 | 22 | |||||||||
| 19 | Carbofuran | C12H15NO3 | [M + H]+ | 3.42 | 222.1 | 123.0 | 22 | 12 | 0.71 | 2.38 | 5–1000 | 0.9991 |
| 222.1 | 165.1 | 22 | 12 | |||||||||
| 20 | Carbosulfan | C20H32N2O3S | [M + H]+ | 4.27 | 381.1 | 117.9 | 24 | 18 | 0.96 | 3.20 | 5–1000 | 0.9987 |
| 381.1 | 159.9 | 24 | 18 | |||||||||
| 21 | Cartap | C7H15N3O2S2 | [M + H]+ | 0.63 | 238.0 | 61.1 | 25 | 22 | 1.57 | 5.22 | 10–1000 | 0.9999 |
| 238.0 | 105.0 | 25 | 16 | |||||||||
| 22 | Chlorantraniliprole | C18H14BRCl2N5O2 | [M + H]+ | 4.20 | 484.1 | 286.1 | 25 | 20 | 0.42 | 1.39 | 5–1000 | 0.9998 |
| 484.1 | 453.0 | 25 | 18 | |||||||||
| 23 | Chlorfenvinphos | C12H14Cl3O4P | [M − H]− | 5.30 | 358.9 | 99.0 | 20 | 30 | 0.96 | 3.22 | 5–1000 | 0.9989 |
| 358.9 | 155.0 | 20 | 12 | |||||||||
| 24 | Chlorpropham | C10H12ClNO2 | [M + H]+ | 4.85 | 214.6 | 126.0 | 20 | 15 | 0.72 | 2.41 | 5–1000 | 0.9983 |
| 214.6 | 154.0 | 20 | 10 | |||||||||
| 25 | Clodinafop-propargyl | C17H13ClFNO4 | [M + H]+ | 6.78 | 350.0 | 266.0 | 22 | 20 | 0.86 | 2.87 | 5–1000 | 0.9997 |
| 350.0 | 91.0 | 22 | 18 | |||||||||
| 26 | Clothianidin | C6H8ClN5O2S | [M + H]+ | 2.16 | 249.9 | 132.0 | 22 | 15 | 0.22 | 0.74 | 2–1000 | 0.9995 |
| 249.9 | 169.0 | 22 | 10 | |||||||||
| 27 | Cyantraniliprole | C19H14BrClN6O2 | [M + H]+ | 3.77 | 475.0 | 286.0 | 25 | 15 | 0.31 | 1.03 | 5–1000 | 0.9995 |
| 475.0 | 444.0 | 25 | 15 | |||||||||
| 28 | Cyazofamid | C13H13ClN4O2S | [M + H]+ | 5.34 | 325.0 | 107.9 | 20 | 20 | 0.36 | 1.19 | 5–1000 | 0.9991 |
| 325.0 | 261.0 | 20 | 10 | |||||||||
| 29 | Cymoxanil | C7H10N4O3 | [M + H]+ | 2.55 | 199.0 | 111.0 | 15 | 18 | 0.86 | 2.87 | 5–1000 | 0.9989 |
| 199.0 | 128.0 | 15 | 8 | |||||||||
| 30 | Cyproconazole | C15H18ClN3O | [M + H]+ | 5.32 | 292.2 | 70.2 | 30 | 18 | 0.22 | 0.73 | 2–1000 | 0.9996 |
| 292.2 | 125.1 | 30 | 24 | |||||||||
| 31 | Cyprodinil | C14H15N3 | [M + H]+ | 4.09 | 226.0 | 93.0 | 30 | 26 | 0.84 | 2.81 | 5–1000 | 0.9992 |
| 226.0 | 108.0 | 30 | 20 | |||||||||
| 32 | Cyromazine | C6H10N6 | [M + H]+ | 2.66 | 167.2 | 60.0 | 28 | 20 | 1.03 | 3.43 | 5–1000 | 0.9992 |
| 167.2 | 108.0 | 28 | 20 | |||||||||
| 33 | Diazinon | C12H21N2O3PS | [M + H]+ | 6.99 | 305.1 | 96.9 | 30 | 35 | 0.63 | 2.10 | 5–1000 | 0.9990 |
| 305.1 | 169.0 | 30 | 22 | |||||||||
| 34 | Diethofencarb | C14H21NO4 | [M + H]+ | 4.49 | 268.3 | 124.0 | 19 | 40 | 0.26 | 0.86 | 2–500 | 0.9989 |
| 268.3 | 226.0 | 19 | 10 | |||||||||
| 35 | Difenoconazole | C19H17Cl2N3O3 | [M + H]+ | 5.40 | 406.0 | 111.1 | 20 | 60 | 0.60 | 1.99 | 5–1000 | 0.9994 |
| 406.0 | 251.1 | 20 | 25 | |||||||||
| 36 | Dimethoate | C5H12NO3PS2 | [M + H]+ | 2.25 | 230.1 | 125.0 | 22 | 10 | 0.40 | 1.34 | 5–1000 | 0.9991 |
| 230.1 | 199.0 | 22 | 6 | |||||||||
| 37 | Dimethomorph | C21H22ClNO4 | [M + H]+ | 4.25 | 388.1 | 165.0 | 35 | 30 | 0.20 | 0.65 | 5–1000 | 0.9994 |
| 388.1 | 300.9 | 35 | 20 | |||||||||
| 38 | Diniconazole | C15H17Cl2N3O | [M + H]+ | 6.30 | 326.1 | 70.2 | 37 | 25 | 0.71 | 2.38 | 5–1000 | 0.9994 |
| 326.1 | 159.0 | 37 | 34 | |||||||||
| 39 | Dinotefuran | C7H14N4O3 | [M + H]+ | 1.00 | 203.0 | 129.0 | 13 | 12 | 0.86 | 2.88 | 5–1000 | 0.9983 |
| 203.0 | 156.9 | 13 | 6 | |||||||||
| 40 | Diuron | C9H10Cl2N2O | [M + H]+ | 3.76 | 233.0 | 46.3 | 28 | 14 | 0.27 | 0.91 | 5–1000 | 0.9974 |
| 233.0 | 72.1 | 28 | 18 | |||||||||
| 41 | Epoxiconazole | C17H13ClFN3O | [M + H]+ | 4.68 | 330.0 | 101.0 | 25 | 50 | 0.55 | 1.84 | 5–1000 | 0.9997 |
| 330.0 | 121.0 | 25 | 22 | |||||||||
| 42 | Ethion | C9H22O4P2S4 | [M + H]+ | 8.05 | 384.9 | 97.0 | 30 | 46 | 0.80 | 2.66 | 5–1000 | 0.9994 |
| 384.9 | 199.1 | 30 | 10 | |||||||||
| 43 | Fenbuconazole | C19H17ClN4 | [M + H]+ | 4.89 | 337.0 | 70.1 | 32 | 20 | 0.24 | 0.82 | 2–500 | 0.9990 |
| 337.0 | 125.0 | 32 | 36 | |||||||||
| 44 | Fenhexamid | C14H17Cl2NO2 | [M + H]+ | 4.74 | 302.1 | 55.3 | 32 | 38 | 0.34 | 1.15 | 5–1000 | 0.9998 |
| 302.1 | 97.2 | 32 | 22 | |||||||||
| 45 | Fenobucarb | C12H17NO2 | [M + H]+ | 4.38 | 208.0 | 94.9 | 16 | 14 | 0.87 | 2.89 | 5–1000 | 0.9966 |
| 208.0 | 152.0 | 16 | 8 | |||||||||
| 46 | Flonicamid | C9H6F3N3O | [M + H]+ | 1.78 | 230.2 | 148.1 | 35 | 25 | 1.36 | 4.52 | 5–1000 | 0.9992 |
| 230.2 | 203.1 | 35 | 15 | |||||||||
| 47 | Fluazifop-butyl | C19H20F3NO4 | [M + H]+ | 6.32 | 384.1 | 282.1 | 28 | 20 | 0.45 | 1.49 | 5–1000 | 0.9985 |
| 384.1 | 328.1 | 28 | 14 | |||||||||
| 48 | Fluazinam | C13H4Cl2F6N4O4 | [M + H]+ | 6.17 | 465.0 | 338.1 | 23 | 47 | 0.27 | 0.91 | 5–1000 | 0.9997 |
| 465.0 | 373.0 | 23 | 26 | |||||||||
| 49 | Fluopicolide | C14H8Cl3F3N2O | [M + H]+ | 4.82 | 386.2 | 173.0 | 35 | 25 | 0.43 | 1.45 | 5–1000 | 0.9995 |
| 386.2 | 175.0 | 35 | 25 | |||||||||
| 50 | Flusilazole | C16H15F2N3Si | [M + H]+ | 5.93 | 316.0 | 165.0 | 30 | 28 | 0.42 | 1.39 | 5–1000 | 0.9993 |
| 316.0 | 247.0 | 30 | 18 | |||||||||
| 51 | Flutriafol | C16H13F2N3O | [M + H]+ | 4.24 | 302.1 | 70.2 | 30 | 18 | 0.14 | 0.46 | 2–1000 | 0.9999 |
| 302.1 | 123.1 | 30 | 29 | |||||||||
| 52 | Fosthiazate | C9H18NO3PS2 | [M + H]+ | 3.63 | 284.0 | 104.0 | 20 | 18 | 0.20 | 0.68 | 2–500 | 0.9998 |
| 284.0 | 228.0 | 20 | 6 | |||||||||
| 53 | Haloxyfop-methyl | C16H13ClF3NO4 | [M + H]+ | 5.82 | 376.0 | 91.1 | 25 | 25 | 0.23 | 0.77 | 5–1000 | 0.9998 |
| 376.0 | 316.1 | 25 | 10 | |||||||||
| 54 | Hexaconazole | C14H17Cl2N3O | [M + H]+ | 6.05 | 315.0 | 70.1 | 31 | 22 | 0.46 | 1.52 | 5–1000 | 0.9992 |
| 315.0 | 159.0 | 31 | 28 | |||||||||
| 55 | Hexythiazox | C17H21ClN2O2S | [M + H]+ | 6.53 | 353.0 | 168.1 | 24 | 26 | 0.90 | 2.98 | 5–1000 | 0.9995 |
| 353.0 | 228.1 | 24 | 14 | |||||||||
| 56 | Imazalil | C14H14Cl2N2O | [M + H]+ | 2.93 | 296.9 | 158.9 | 25 | 20 | 0.60 | 2.00 | 5–1000 | 0.9999 |
| 296.9 | 201.0 | 25 | 15 | |||||||||
| 57 | Imidacloprid | C9H10ClN5O2 | [M + H]+ | 2.13 | 256.1 | 175.1 | 22 | 20 | 0.31 | 1.02 | 5–1000 | 0.9999 |
| 256.1 | 209.1 | 22 | 15 | |||||||||
| 58 | Indoxacarb | C22H17ClF3N3O7 | [M + H]+ | 5.83 | 528.0 | 150.0 | 25 | 22 | 0.37 | 1.23 | 5–1000 | 0.9993 |
| 528.0 | 203.0 | 25 | 40 | |||||||||
| 59 | Iprodione | C13H13Cl2N3O3 | [M + H]+ | 4.98 | 330.0 | 244.7 | 15 | 16 | 1.57 | 5.23 | 10–1000 | 0.9995 |
| 330.0 | 288.0 | 15 | 15 | |||||||||
| 60 | Isocarbophos | C11H16NO4PS | [M + H]+ | 4.30 | 291.1 | 121.1 | 12 | 30 | 0.21 | 0.70 | 2–500 | 0.9992 |
| 291.1 | 231.1 | 12 | 13 | |||||||||
| 61 | Isoprocarb | C11H15NO2 | [M + H]+ | 3.91 | 194.1 | 95.1 | 18 | 14 | 0.33 | 1.09 | 5–1000 | 0.9981 |
| 194.1 | 137.1 | 18 | 8 | |||||||||
| 62 | Isoprothiolane | C12H18O4S2 | [M + H]+ | 5.04 | 291.1 | 188.8 | 15 | 18 | 0.22 | 0.72 | 5–1000 | 0.9991 |
| 291.1 | 230.9 | 15 | 10 | |||||||||
| 63 | Isoproturon | C12H18N2O | [M + H]+ | 3.72 | 207.0 | 72.0 | 28 | 22 | 0.30 | 0.99 | 5–1000 | 0.9982 |
| 207.0 | 165.1 | 28 | 15 | |||||||||
| 64 | Malathion | C10H19O6PS2 | [M + H]+ | 4.99 | 331.0 | 99.0 | 14 | 24 | 0.21 | 0.71 | 5–1000 | 0.9998 |
| 331.0 | 127.0 | 14 | 12 | |||||||||
| 65 | Metalaxyl | C15H21NO4 | [M + H]+ | 4.49 | 280.1 | 192.1 | 30 | 17 | 0.21 | 0.70 | 2–1000 | 0.9997 |
| 280.1 | 220.1 | 30 | 13 | |||||||||
| 66 | Metconazole | C17H22ClN3O | [M + H]+ | 5.05 | 320.1 | 70.0 | 25 | 22 | 0.52 | 1.73 | 5–1000 | 0.9993 |
| 320.1 | 125.0 | 25 | 36 | |||||||||
| 67 | Methamidophos | C2H8NO2PS | [M + H]+ | 0.74 | 142.0 | 94.0 | 22 | 10 | 0.44 | 1.48 | 5–1000 | 0.9999 |
| 142.0 | 124.9 | 22 | 10 | |||||||||
| 68 | Methomyl | C5H10N2O2S | [M + H]+ | 1.51 | 163.0 | 88.0 | 20 | 10 | 0.32 | 1.06 | 5–1000 | 0.9992 |
| 163.0 | 106.0 | 20 | 10 | |||||||||
| 69 | Methoxyfenozide | C22H28N2O3 | [M + H]+ | 4.90 | 369.1 | 149.1 | 25 | 18 | 0.53 | 1.78 | 5–1000 | 0.9994 |
| 369.1 | 313.2 | 25 | 8 | |||||||||
| 70 | Metolachlor | C15H22ClNO2 | [M + H]+ | 6.27 | 284.1 | 176.1 | 30 | 25 | 0.40 | 1.35 | 5–1000 | 0.9997 |
| 284.1 | 252.1 | 30 | 15 | |||||||||
| 71 | Metribuzin | C8H14N4OS | [M + H]+ | 3.86 | 215.0 | 131.0 | 10 | 18 | 0.62 | 2.07 | 5–1000 | 0.9987 |
| 215.0 | 89.0 | 20 | 16 | |||||||||
| 72 | Myclobutanil | C15H17ClN4 | [M + H]+ | 4.59 | 289.1 | 70.2 | 28 | 18 | 0.35 | 1.18 | 5–1000 | 0.9992 |
| 289.1 | 125.1 | 28 | 32 | |||||||||
| 73 | Omethoate | C5H12NO4PS | [M + H]+ | 0.83 | 214.0 | 125.0 | 20 | 22 | 0.63 | 2.09 | 5–1000 | 0.9983 |
| 214.0 | 183.0 | 20 | 11 | |||||||||
| 74 | Paclobutrazol | C15H20ClN3O | [M + H]+ | 5.15 | 294.1 | 70.2 | 30 | 20 | 0.40 | 1.34 | 5–1000 | 0.9995 |
| 294.1 | 125.1 | 30 | 38 | |||||||||
| 75 | Penconazole | C13H15Cl2N3 | [M + H]+ | 4.99 | 284.0 | 70.1 | 28 | 16 | 0.38 | 1.26 | 5–1000 | 0.9999 |
| 284.0 | 159.0 | 28 | 34 | |||||||||
| 76 | Phenthoate | C12H17O4PS2 | [M + H]+ | 6.88 | 321.0 | 135.0 | 30 | 20 | 1.91 | 6.37 | 10–1000 | 0.9999 |
| 321.0 | 163.0 | 30 | 12 | |||||||||
| 77 | Phorate | C7H17O2PS3 | [M + H]+ | 5.82 | 261.0 | 75.0 | 10 | 12 | 1.62 | 5.40 | 10–1000 | 0.9949 |
| 78 | Phorate-sulfone | C7H17O4PS3 | [M + NH4]+ | 4.29 | 293.0 | 115.0 | 16 | 24 | 0.51 | 1.71 | 5–1000 | 0.9979 |
| 293.0 | 171.0 | 16 | 6 | |||||||||
| 79 | Phorate-sulfoxide | C7H17O3PS3 | [M + H]+ | 3.55 | 277.0 | 96.9 | 18 | 32 | 0.15 | 0.50 | 2–500 | 0.9989 |
| 277.0 | 143.0 | 18 | 20 | |||||||||
| 80 | Phoxim | C12H15N2O3PS | [M + H]+ | 5.77 | 299.0 | 129.0 | 12 | 13 | 0.45 | 1.49 | 5–1000 | 0.9977 |
| 299.0 | 153.0 | 12 | 7 | |||||||||
| 81 | Piperonyl-butoxide | C19H30O5 | [M + NH4]+ | 6.27 | 356.3 | 119.0 | 20 | 30 | 0.57 | 1.91 | 5–1000 | 0.9999 |
| 356.3 | 176.9 | 20 | 10 | |||||||||
| 82 | Pirimiphos-methyl | C11H20N3O3PS | [M + H]+ | 6.90 | 306.1 | 108.1 | 30 | 32 | 0.55 | 1.84 | 5–1000 | 0.9991 |
| 306.1 | 164.1 | 30 | 22 | |||||||||
| 83 | Prochloraz | C15H16Cl3N3O2 | [M + H]+ | 9.87 | 376.1 | 266.0 | 22 | 10 | 0.89 | 2.97 | 5–1000 | 0.9978 |
| 376.1 | 308.0 | 22 | 10 | |||||||||
| 84 | Profenofos | C11H15BrClO3PS | [M − H]− | 7.40 | 372.9 | 127.9 | 30 | 40 | 1.14 | 3.80 | 5–1000 | 0.9999 |
| 372.9 | 302.6 | 30 | 20 | |||||||||
| 85 | Prometryn | C10H19N5S | [M + H]+ | 3.45 | 242.0 | 158.0 | 20 | 16 | 0.69 | 2.31 | 5–1000 | 0.9995 |
| 242.0 | 200.1 | 20 | 12 | |||||||||
| 86 | Propham | C10H13NO2 | [M + H]+ | 4.07 | 180.0 | 77.0 | 18 | 20 | 0.26 | 0.88 | 2–1000 | 0.9991 |
| 180.0 | 120.0 | 18 | 10 | |||||||||
| 87 | Propiconazole | C15H17Cl2N3O2 | [M + H]+ | 5.15 | 342.0 | 69.0 | 25 | 20 | 0.16 | 0.54 | 2–500 | 0.9996 |
| 342.0 | 159.0 | 25 | 30 | |||||||||
| 88 | Propoxur | C11H15NO3 | [M + H]+ | 3.36 | 210.0 | 111.0 | 12 | 16 | 0.44 | 1.45 | 5–1000 | 0.9968 |
| 89 | Pyridaben | C19H25ClN2OS | [M + H]+ | 7.00 | 365.1 | 147.0 | 22 | 20 | 0.24 | 0.80 | 5–1000 | 0.9985 |
| 365.1 | 309.1 | 22 | 8 | |||||||||
| 90 | Semiamitraz | C10H14N2 | [M + H]+ | 3.94 | 163.0 | 96.3 | 18 | 10 | 1.32 | 4.40 | 5–1000 | 0.9921 |
| 163.0 | 118.4 | 18 | 10 | |||||||||
| 91 | Sethoxydim | C17H29NO3S | [M + H]+ | 6.42 | 328.3 | 254.3 | 23 | 15 | 1.50 | 4.99 | 5–1000 | 0.9976 |
| 328.3 | 282.0 | 23 | 10 | |||||||||
| 92 | Simazine | C7H12ClN5 | [M + H]+ | 3.47 | 202.0 | 96.0 | 25 | 20 | 0.30 | 0.99 | 5–1000 | 0.9999 |
| 202.0 | 124.0 | 25 | 14 | |||||||||
| 93 | Spirodiclofen | C21H24Cl2O4 | [M + H]+ | 7.10 | 411.1 | 71.2 | 25 | 13 | 0.78 | 2.61 | 5–1000 | 0.9997 |
| 411.1 | 313.0 | 25 | 13 | |||||||||
| 94 | Tebuconazole | C16H22ClN3O | [M + H]+ | 4.82 | 308.0 | 70.1 | 34 | 22 | 0.24 | 0.79 | 5–1000 | 0.9996 |
| 308.0 | 125.0 | 34 | 40 | |||||||||
| 95 | Tebufenozide | C22H28N2O2 | [M + H]+ | 5.25 | 353.0 | 105.0 | 12 | 22 | 0.28 | 0.95 | 5–1000 | 0.9996 |
| 353.0 | 133.0 | 12 | 18 | |||||||||
| 96 | Thiabendazole | C10H7N3S | [M + H]+ | 1.03 | 202.0 | 131.0 | 25 | 25 | 0.30 | 0.99 | 5–1000 | 0.9998 |
| 202.0 | 175.0 | 25 | 20 | |||||||||
| 97 | Thiacloprid | C10H9ClN4S | [M + H]+ | 2.69 | 253.0 | 90.1 | 25 | 30 | 0.20 | 0.67 | 5–1000 | 0.9997 |
| 253.0 | 126.0 | 25 | 10 | |||||||||
| 98 | Thiamethoxam | C8H10ClN5O3S | [M + H]+ | 1.68 | 292.0 | 132.0 | 22 | 22 | 0.46 | 1.53 | 5–1000 | 0.9999 |
| 292.0 | 211.2 | 22 | 12 | |||||||||
| 99 | Thifluzamide | C13H6Br2F6N2O2S | [M + H]+ | 6.53 | 526.8 | 148.0 | 20 | 25 | 1.69 | 5.63 | 10–1000 | 0.9993 |
| 526.8 | 168.0 | 20 | 25 | |||||||||
| 100 | Thiophanate-methyl | C12H14N4O4S2 | [M + H]+ | 3.20 | 343.1 | 93.0 | 20 | 46 | 0.72 | 2.39 | 5–1000 | 0.9996 |
| 343.1 | 151.0 | 20 | 22 | |||||||||
| 101 | Tolfenpyrad | C21H22ClN3O2 | [M + H]+ | 6.11 | 384.2 | 171.0 | 30 | 20 | 1.13 | 3.78 | 5–1000 | 0.9997 |
| 384.2 | 197.0 | 30 | 18 | |||||||||
| 102 | Triadimefon | C14H16ClN3O2 | [M + H]+ | 5.74 | 294.1 | 69.3 | 30 | 20 | 0.20 | 0.68 | 5–1000 | 0.9988 |
| 294.1 | 197.2 | 30 | 15 | |||||||||
| 103 | Triazophos | C12H16N3O3PS | [M + H]+ | 6.19 | 314.1 | 118.9 | 30 | 35 | 0.49 | 1.63 | 5–1000 | 0.9996 |
| 314.1 | 161.9 | 30 | 18 | |||||||||
| 104 | Tribenuron-methyl | C15H17N5O6S | [M + H]+ | 4.09 | 396.1 | 154.9 | 18 | 14 | 0.30 | 1.00 | 5–1000 | 0.9885 |
| 396.1 | 180.9 | 18 | 22 | |||||||||
| 105 | Tridemorph | C19H39NO | [M + H]+ | 4.29 | 298.1 | 57.0 | 40 | 28 | 1.24 | 4.14 | 5–1000 | 0.9997 |
| 298.1 | 98.0 | 40 | 34 | |||||||||
| 106 | Trifloxystrobin | C20H19F3N2O4 | [M + H]+ | 5.94 | 409.0 | 145.0 | 25 | 25 | 0.48 | 1.58 | 5–1000 | 0.9994 |
| 409.0 | 186.0 | 25 | 8 | |||||||||
| 107 | Triflumizole | C15H15ClF3N3O | [M + H]+ | 4.92 | 346.0 | 277.9 | 13 | 10 | 1.75 | 5.82 | 10–1000 | 0.9998 |
| 359.0 | 139.1 | 20 | 35 |
| No. | Compound | 10 µg/kg | 50 µg/kg | 100 µg/kg | Intra-day Precision (RSD%, n = 5) | Inter-day Precision (RSD%, n = 5) | Stability (RSD, %) | ME (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rec. (%) | RSD (%) | Rec. (%) | RSD (%) | Rec. (%) | RSD (%) | 10 µg/kg | 100 µg/kg | 10 µg/kg | 100 µg/kg | 100 µg/kg | 10 µg/kg | ||
| 1 | Abamectin | 83.3 | 8.1 | 86.0 | 13.1 | 87.0 | 8.2 | 3.2 | 2.7 | 5.4 | 1.9 | 6.2 | 90.0 |
| 2 | Acephate | 71.7 | 1.0 | 80.0 | 2.0 | 67.0 | 0.9 | 0.7 | 3.1 | 3.6 | 6.3 | 5.4 | 83.8 |
| 3 | Acetamiprid | 85.0 | 8.8 | 96.0 | 9.0 | 93.5 | 6.8 | 1.6 | 1.6 | 2.1 | 2.8 | 3.9 | 97.5 |
| 4 | Acetochlor | 86.7 | 7.9 | 104.3 | 5.9 | 102.3 | 3.3 | 2.1 | 5.4 | 6.3 | 4.6 | 4.8 | 99.6 |
| 5 | Aldicarb | 96.7 | 5.6 | 92.3 | 3.7 | 86.8 | 0.6 | 6.2 | 1.0 | 4.8 | 11.7 | 1.9 | 92.4 |
| 6 | Aldicarb-Sulfone | 90.0 | 13.5 | 93.3 | 10.4 | 94.7 | 6.6 | 0.6 | 2.2 | 7.2 | 2.7 | 7.2 | 91.6 |
| 7 | Aldicarb-Sulfoxide | 85.0 | 5.5 | 78.0 | 4.1 | 102.0 | 4.7 | 0.8 | 3.6 | 1.9 | 10.8 | 10.8 | 87.9 |
| 8 | Ametryn | 90.0 | 2.7 | 92.3 | 5.7 | 102.0 | 3.6 | 2.3 | 7.8 | 4.5 | 11.6 | 3.5 | 101.4 |
| 9 | Atrazine | 103.3 | 9.1 | 92.0 | 4.1 | 96.5 | 5.3 | 5.7 | 1.2 | 2.6 | 3.8 | 7.2 | 102.6 |
| 10 | Azoxystrobin | 63.3 | 9.1 | 77.3 | 2.6 | 104.7 | 2.7 | 1.0 | 4.3 | 5.6 | 4.6 | 5.6 | 96.8 |
| 11 | Bendiocarb | 103.3 | 2.8 | 104.6 | 4.3 | 92.8 | 0.6 | 9.3 | 3.2 | 10.2 | 7.2 | 6.3 | 104.7 |
| 12 | Blasticidin-S | 96.5 | 3.1 | 101.2 | 1.6 | 99.8 | 0.7 | 5.6 | 7.6 | 2.8 | 6.3 | 8.2 | 103.2 |
| 13 | Boscalid | 80.0 | 10.8 | 79.0 | 7.4 | 77.0 | 3.3 | 7.2 | 4.5 | 4.6 | 2.1 | 6.3 | 79.8 |
| 14 | Bupirimate | 116.0 | 8.9 | 93.7 | 5.2 | 93.2 | 4.3 | 1.4 | 6.2 | 7.9 | 1.6 | 4.9 | 107.2 |
| 15 | Buprofezin | 103.3 | 5.6 | 98.0 | 2.0 | 85.8 | 0.9 | 5.3 | 1.9 | 5.3 | 1.9 | 5.1 | 92.4 |
| 16 | Butralin | 91.7 | 8.3 | 92.6 | 0.6 | 85.0 | 3.8 | 5.9 | 8.2 | 4.1 | 2.0 | 7.2 | 88.3 |
| 17 | Carbaryl | 103.3 | 12.9 | 79.0 | 5.0 | 92.0 | 2.4 | 2.7 | 4.1 | 2.2 | 3.6 | 4.1 | 94.5 |
| 18 | Carbendazim | 92.4 | 6.3 | 96.3 | 3.2 | 102.6 | 1.8 | 0.5 | 2.0 | 1.0 | 5.4 | 2.8 | 95.2 |
| 19 | Carbofuran | 113.3 | 2.5 | 118.6 | 6.2 | 118.3 | 3.3 | 9.4 | 3.3 | 3.2 | 8.2 | 2.2 | 121.6 |
| 20 | Carbosulfan | 106.3 | 2.1 | 98.2 | 1.9 | 104.2 | 4.1 | 1.6 | 6.1 | 5.1 | 7.5 | 1.6 | 98.2 |
| 21 | Cartap | 108.3 | 2.6 | 85.6 | 3.5 | 89.0 | 6.1 | 4.1 | 2.9 | 11.3 | 1.9 | 4.3 | 95.1 |
| 22 | Chlorantraniliprole | 100.0 | 13.2 | 82.5 | 4.5 | 79.0 | 6.7 | 6.8 | 2.5 | 0.9 | 3.6 | 5.3 | 89.7 |
| 23 | Chlorfenvinphos | 95.0 | 3.6 | 88.7 | 5.5 | 89.7 | 3.9 | 2.4 | 1.8 | 2.8 | 9.2 | 5.9 | 92.3 |
| 24 | Chlorpropham | 100.0 | 5.0 | 106.7 | 10.1 | 75.3 | 13.6 | 2.2 | 5.5 | 5.6 | 1.6 | 11.2 | 80.6 |
| 25 | Clodinafop-propargyl | 83.3 | 3.4 | 86.0 | 11.0 | 85.8 | 7.3 | 1.0 | 4.9 | 8.4 | 5.5 | 9.7 | 87.1 |
| 26 | Clothianidin | 88.3 | 8.6 | 90.3 | 3.5 | 104.5 | 6.3 | 1.3 | 5.6 | 7.6 | 8.0 | 3.8 | 93.5 |
| 27 | Cyantraniliprole | 75.0 | 13.3 | 77.5 | 4.8 | 83.8 | 5.6 | 3.0 | 8.2 | 3.5 | 7.9 | 4.9 | 85.9 |
| 28 | Cyazofamid | 100.0 | 3.1 | 86.7 | 2.4 | 94.5 | 1.9 | 1.8 | 7.1 | 6.1 | 3.6 | 7.4 | 96.1 |
| 29 | Cymoxanil | 115.0 | 11.5 | 99.0 | 6.0 | 96.3 | 3.5 | 2.6 | 2.3 | 4.8 | 2.1 | 6.6 | 104.2 |
| 30 | Cyproconazole | 96.7 | 2.9 | 87.7 | 1.7 | 103.2 | 6.1 | 2.8 | 1.1 | 6.6 | 9.7 | 5.9 | 95.3 |
| 31 | Cyprodinil | 80.0 | 12.7 | 78.3 | 10.3 | 88.9 | 11.8 | 4.2 | 1.0 | 7.4 | 4.8 | 8.7 | 88.9 |
| 32 | Cyromazine | 90.7 | 6.8 | 95.4 | 3.2 | 94.6 | 5.7 | 3.1 | 2.0 | 2.5 | 5.1 | 9.2 | 90.2 |
| 33 | Diazinon | 96.7 | 2.9 | 97.3 | 0.5 | 96.7 | 1.6 | 6.5 | 2.4 | 4.6 | 3.2 | 7.4 | 100.4 |
| 34 | Diethofencarb | 100.0 | 5.0 | 97.3 | 4.2 | 114.0 | 7.0 | 5.1 | 3.5 | 1.9 | 1.9 | 5.4 | 106.2 |
| 35 | Difenoconazole | 75.0 | 6.7 | 75.7 | 2.5 | 87.8 | 4.4 | 3.1 | 6.1 | 8.4 | 5.6 | 3.2 | 90.3 |
| 36 | Dimethoate | 95.0 | 2.6 | 97.0 | 2.0 | 92.2 | 0.8 | 5.0 | 2.2 | 7.6 | 4.9 | 2.6 | 94.8 |
| 37 | Dimethomorph | 86.7 | 3.3 | 92.0 | 1.0 | 90.3 | 4.9 | 6.2 | 0.8 | 3.8 | 8.5 | 2.1 | 91.7 |
| 38 | Diniconazole | 76.7 | 3.7 | 79.0 | 4.8 | 76.3 | 7.3 | 1.8 | 1.2 | 5.4 | 4.0 | 4.4 | 86.3 |
| 39 | Dinotefuran | 88.3 | 8.6 | 92.0 | 3.9 | 103.0 | 6.5 | 0.9 | 1.9 | 2.9 | 3.9 | 7.0 | 88.6 |
| 40 | Diuron | 75.0 | 6.6 | 76.0 | 4.8 | 78.3 | 4.4 | 2.2 | 3.7 | 0.8 | 2.1 | 6.2 | 87.2 |
| 41 | Epoxiconazole | 72.0 | 4.0 | 82.5 | 9.4 | 81.5 | 7.1 | 2.3 | 2.6 | 11.6 | 6.3 | 5.9 | 89.1 |
| 42 | Ethion | 78.3 | 13.2 | 96.3 | 4.3 | 89.2 | 7.3 | 1.6 | 5.4 | 12.3 | 9.7 | 7.6 | 93.2 |
| 43 | Fenbuconazole | 80.0 | 2.2 | 83.5 | 5.6 | 81.2 | 10.3 | 1.8 | 5.7 | 2.2 | 8.2 | 3.9 | 91.7 |
| 44 | Fenhexamid | 111.0 | 11.2 | 87.3 | 11.0 | 75.2 | 7.1 | 3.3 | 6.1 | 2.0 | 5.1 | 9.8 | 92.8 |
| 45 | Fenobucarb | 105.0 | 8.2 | 90.7 | 9.1 | 91.7 | 4.7 | 2.9 | 3.3 | 6.5 | 4.2 | 11.7 | 99.7 |
| 46 | Flonicamid | 101.7 | 2.8 | 93.0 | 2.8 | 103.0 | 2.7 | 4.2 | 1.8 | 1.9 | 1.2 | 7.9 | 103.2 |
| 47 | Fluazifop-butyl | 88.3 | 6.5 | 95.7 | 2.1 | 84.5 | 4.3 | 3.6 | 1.1 | 7.1 | 2.9 | 5.9 | 96.5 |
| 48 | Fluazinam | 81.7 | 9.3 | 82.0 | 12.2 | 76.7 | 5.5 | 5.1 | 2.6 | 6.3 | 3.5 | 4.2 | 86.7 |
| 49 | Fluopicolide | 83.3 | 3.4 | 93.3 | 7.5 | 100.8 | 3.6 | 1.8 | 3.7 | 1.8 | 7.4 | 1.8 | 96.3 |
| 50 | Flusilazole | 93.3 | 3.0 | 93.0 | 2.1 | 91.8 | 5.8 | 1.7 | 8.6 | 7.7 | 6.1 | 4.1 | 97.2 |
| 51 | Flutriafol | 88.3 | 3.2 | 91.0 | 4.7 | 106.8 | 5.1 | 2.2 | 9.0 | 6.5 | 9.8 | 3.1 | 95.4 |
| 52 | Fosthiazate | 96.7 | 7.9 | 96.3 | 0.6 | 93.8 | 2.1 | 1.3 | 5.7 | 13.6 | 4.2 | 5.8 | 100.7 |
| 53 | Haloxyfop-methyl | 91.7 | 3.1 | 88.3 | 3.6 | 87.7 | 5.7 | 3.3 | 3.1 | 5.8 | 6.6 | 4.2 | 90.6 |
| 54 | Hexaconazole | 86.7 | 12.0 | 92.7 | 8.3 | 101.7 | 6.4 | 2.9 | 6.8 | 6.2 | 1.5 | 1.9 | 95.7 |
| 55 | Hexythiazox | 75.0 | 6.6 | 89.0 | 2.9 | 91.0 | 10.0 | 8.2 | 7.2 | 4.9 | 4.6 | 7.4 | 77.3 |
| 56 | Imazalil | 68.3 | 11.1 | 80.0 | 8.0 | 70.2 | 3.1 | 7.6 | 1.9 | 7.5 | 5.1 | 4.8 | 85.9 |
| 57 | Imidacloprid | 76.7 | 3.7 | 86.3 | 2.6 | 102.3 | 2.2 | 1.4 | 4.4 | 4.6 | 8.4 | 4.6 | 94.3 |
| 58 | Indoxacarb | 88.3 | 3.2 | 93.7 | 4.0 | 82.3 | 9.9 | 6.2 | 4.7 | 1.8 | 7.4 | 5.0 | 91.6 |
| 59 | Iprodione | 90.0 | 5.5 | 72.0 | 2.7 | 86.8 | 9.2 | 9.4 | 2.8 | 4.2 | 3.3 | 2.7 | 87.8 |
| 60 | Isocarbophos | 80.0 | 12.5 | 96.0 | 8.1 | 96.3 | 6.2 | 8.0 | 3.9 | 1.3 | 2.9 | 6.0 | 98.4 |
| 61 | Isoprocarb | 106.7 | 2.7 | 95.0 | 4.8 | 93.7 | 1.3 | 7.2 | 6.5 | 2.6 | 6.1 | 4.8 | 103.2 |
| 62 | Isoprothiolane | 115.0 | 11.5 | 105.0 | 4.3 | 89.2 | 7.7 | 4.4 | 2.2 | 5.8 | 3.2 | 7.1 | 101.6 |
| 63 | Isoproturon | 101.7 | 2.8 | 94.7 | 0.6 | 106.0 | 3.1 | 4.6 | 1.3 | 7.1 | 1.9 | 4.2 | 99.1 |
| 64 | Malathion | 80.0 | 2.3 | 87.3 | 1.1 | 89.5 | 2.1 | 2.8 | 7.2 | 6.3 | 7.8 | 6.6 | 96.7 |
| 65 | Metalaxyl | 90.0 | 5.5 | 97.3 | 2.1 | 93.5 | 1.4 | 3.9 | 4.5 | 6.9 | 6.4 | 5.9 | 92.5 |
| 66 | Metconazole | 76.7 | 13.5 | 76.0 | 5.9 | 73.2 | 5.5 | 4.1 | 6.1 | 5.5 | 1.4 | 8.2 | 76.3 |
| 67 | Methamidophos | 76.7 | 3.7 | 81.0 | 2.4 | 96.7 | 3.0 | 3.2 | 2.8 | 1.8 | 0.8 | 8.0 | 88.2 |
| 68 | Methomyl | 88.3 | 11.7 | 95.0 | 4.5 | 115.8 | 3.2 | 6.1 | 7.0 | 8.2 | 1.9 | 10.6 | 98.4 |
| 69 | Methoxyfenozide | 85.0 | 11.7 | 87.7 | 9.2 | 90.3 | 3.3 | 5.8 | 8.2 | 3.6 | 2.8 | 2.1 | 91.5 |
| 70 | Metolachlor | 100.0 | 1.8 | 97.7 | 3.1 | 95.8 | 2.6 | 4.6 | 9.1 | 5.8 | 4.6 | 1.9 | 96.7 |
| 71 | Metribuzin | 91.7 | 6.3 | 94.0 | 6.5 | 93.5 | 3.2 | 0.8 | 2.4 | 6.2 | 7.1 | 1.5 | 89.7 |
| 72 | Myclobutanil | 118.0 | 6.4 | 93.7 | 2.2 | 105.7 | 4.9 | 1.1 | 3.3 | 2.9 | 7.3 | 4.1 | 102.7 |
| 73 | Omethoate | 81.7 | 3.5 | 76.3 | 5.4 | 94.2 | 3.2 | 2.3 | 5.1 | 7.1 | 5.9 | 3.3 | 88.2 |
| 74 | Paclobutrazol | 95.0 | 5.2 | 90.0 | 6.7 | 98.8 | 4.3 | 2.9 | 2.9 | 4.4 | 4.5 | 2.3 | 95.4 |
| 75 | Penconazole | 100.0 | 5.0 | 95.0 | 2.8 | 99.5 | 3.5 | 2.2 | 4.6 | 1.8 | 2.9 | 6.5 | 98.7 |
| 76 | Phenthoate | 85.0 | 5.8 | 99.7 | 1.2 | 93.0 | 1.9 | 5.1 | 4.1 | 2.6 | 8.7 | 4.9 | 94.2 |
| 77 | Phorate | 86.7 | 13.8 | 105.3 | 5.5 | 110.1 | 5.1 | 1.6 | 2.9 | 3.7 | 12.6 | 7.3 | 102.1 |
| 78 | Phorate-sulfone | 80.0 | 6.2 | 100.7 | 4.6 | 103.0 | 3.2 | 1.1 | 4.1 | 5.6 | 5.9 | 5.4 | 97.4 |
| 79 | Phorate-sulfoxide | 90.0 | 5.5 | 95.7 | 1.2 | 98.8 | 1.0 | 7.5 | 6.2 | 4.4 | 4.8 | 6.1 | 92.7 |
| 80 | Phoxim | 88.3 | 6.5 | 98.7 | 1.5 | 100.0 | 1.1 | 8.6 | 5.7 | 4.4 | 8.9 | 9.7 | 95.1 |
| 81 | Piperonyl butoxide | 81.7 | 3.5 | 85.0 | 3.1 | 90.7 | 4.2 | 8.3 | 6.0 | 2.8 | 4.6 | 8.2 | 87.3 |
| 82 | Pirimiphos-methyl | 85.0 | 4.1 | 95.7 | 3.7 | 106.1 | 3.0 | 1.9 | 2.8 | 7.6 | 7.6 | 8.9 | 89.6 |
| 83 | Prochloraz | 89.6 | 1.4 | 91.6 | 0.9 | 96.3 | 2.5 | 6.7 | 4.9 | 3.6 | 1.6 | 4.9 | 92.3 |
| 84 | Profenofos | 91.7 | 3.1 | 90.0 | 5.7 | 85.5 | 5.1 | 3.2 | 6.6 | 5.9 | 5.2 | 4.7 | 92.5 |
| 85 | Prometryn | 90.0 | 3.8 | 94.3 | 0.6 | 109.5 | 1.6 | 4.1 | 7.4 | 5.6 | 1.3 | 5.4 | 94.8 |
| 86 | Propham | 116.6 | 2.4 | 89.3 | 6.5 | 92.2 | 3.6 | 8.8 | 2.9 | 3.8 | 5.6 | 8.2 | 108.2 |
| 87 | Propiconazole | 83.3 | 6.9 | 87.0 | 3.0 | 98.7 | 6.9 | 8.4 | 3.6 | 4.2 | 4.3 | 1.7 | 95.1 |
| 88 | Propoxur | 101.7 | 10.2 | 90.0 | 8.9 | 103.5 | 1.0 | 6.2 | 1.9 | 1.9 | 6.3 | 4.6 | 102.7 |
| 89 | Pyridaben | 80.0 | 1.9 | 95.3 | 2.4 | 103.8 | 11.2 | 8.5 | 1.3 | 4.4 | 2.6 | 6.2 | 94.3 |
| 90 | Semiamitraz | 85.6 | 8.9 | 89.3 | 2.6 | 87.6 | 3.5 | 1.9 | 3.5 | 7.5 | 1.9 | 3.9 | 89.7 |
| 91 | Sethoxydim | 123.0 | 8.4 | 101.7 | 4.0 | 90.3 | 4.0 | 2.7 | 4.9 | 6.3 | 4.6 | 7.4 | 107.3 |
| 92 | Simazine | 91.7 | 3.1 | 89.0 | 6.0 | 89.8 | 10.2 | 3.6 | 8.2 | 6.9 | 2.5 | 1.5 | 90.6 |
| 93 | Spirodiclofen | 85.0 | 12.9 | 97.3 | 2.4 | 82.5 | 7.3 | 1.8 | 7.1 | 5.5 | 1.0 | 8.4 | 87.5 |
| 94 | Tebuconazol | 95.0 | 5.2 | 80.0 | 7.6 | 81.0 | 3.2 | 2.2 | 7.5 | 8.0 | 6.8 | 5.6 | 89.4 |
| 95 | Tebufenozide | 111.7 | 8.5 | 99.3 | 7.4 | 84.7 | 2.3 | 2.2 | 2.9 | 10.6 | 7.2 | 9.2 | 98.6 |
| 96 | Tetraconazole | 85.0 | 5.8 | 91.7 | 1.6 | 100.8 | 4.2 | 6.3 | 6.2 | 2.8 | 2.2 | 9.1 | 92.5 |
| 97 | Thiacloprid | 88.3 | 3.2 | 93.7 | 0.6 | 92.7 | 2.1 | 4.1 | 5.9 | 4.5 | 2.6 | 4.8 | 93.6 |
| 98 | Thiamethoxam | 88.3 | 3.2 | 90.0 | 2.9 | 92.7 | 1.1 | 3.9 | 3.8 | 11.3 | 5.1 | 4.3 | 97.4 |
| 99 | Thifluzamide | 71.7 | 4.0 | 103.7 | 8.2 | 89.5 | 8.7 | 5.7 | 1.6 | 1.7 | 4.3 | 2.2 | 88.7 |
| 100 | Thiophanate-methyl | 88.3 | 3.2 | 92.0 | 3.7 | 92.2 | 12.4 | 6.2 | 4.6 | 12.8 | 6.2 | 2.6 | 91.8 |
| 101 | Tolfenpyrad | 83.3 | 9.1 | 80.0 | 3.3 | 84.2 | 2.0 | 2.4 | 1.5 | 3.3 | 1.8 | 9.0 | 86.9 |
| 102 | Triadimefon | 91.7 | 3.1 | 91.7 | 4.9 | 92.0 | 3.8 | 3.3 | 2.3 | 5.7 | 4.1 | 8.1 | 96.5 |
| 103 | Triazophos | 100.0 | 5.0 | 90.7 | 9.2 | 84.7 | 12.7 | 0.8 | 6.2 | 4.9 | 2.9 | 6.1 | 103.6 |
| 104 | Tribenuron-methyl | 100.0 | 3.3 | 73.3 | 13.2 | 82.8 | 2.1 | 1.9 | 5.5 | 8.1 | 3.6 | 7.2 | 89.7 |
| 105 | Tridemorph | 105.0 | 4.7 | 81.7 | 11.4 | 79.5 | 7.7 | 5.1 | 1.9 | 6.2 | 1.1 | 3.6 | 91.8 |
| 106 | Trifloxystrobin | 91.7 | 3.1 | 95.3 | 0.6 | 83.3 | 2.2 | 3.3 | 2.1 | 5.0 | 3.2 | 4.9 | 90.2 |
| 107 | Triflumizole | 90.0 | 5.5 | 86.3 | 8.8 | 95.2 | 4.9 | 2.9 | 4.6 | 4.1 | 5.1 | 6.2 | 93.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-N.; Lian, Y.-J.; Zhou, Y.-R.; Wang, M.-H.; Zhang, X.-Q.; Wang, J.-H.; Wu, Y.-N.; Wang, M.-L. Determination of 107 Pesticide Residues in Wolfberry with Acetate-buffered Salt Extraction and Sin-QuEChERS Nano Column Purification Coupled with Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Molecules 2019, 24, 2918. https://doi.org/10.3390/molecules24162918
Chen J-N, Lian Y-J, Zhou Y-R, Wang M-H, Zhang X-Q, Wang J-H, Wu Y-N, Wang M-L. Determination of 107 Pesticide Residues in Wolfberry with Acetate-buffered Salt Extraction and Sin-QuEChERS Nano Column Purification Coupled with Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Molecules. 2019; 24(16):2918. https://doi.org/10.3390/molecules24162918
Chicago/Turabian StyleChen, Jia-Nan, Yu-Jing Lian, Yi-Ran Zhou, Ming-Hui Wang, Xi-Qing Zhang, Jian-Hua Wang, Yong-Ning Wu, and Ming-Lin Wang. 2019. "Determination of 107 Pesticide Residues in Wolfberry with Acetate-buffered Salt Extraction and Sin-QuEChERS Nano Column Purification Coupled with Ultra Performance Liquid Chromatography Tandem Mass Spectrometry" Molecules 24, no. 16: 2918. https://doi.org/10.3390/molecules24162918
APA StyleChen, J.-N., Lian, Y.-J., Zhou, Y.-R., Wang, M.-H., Zhang, X.-Q., Wang, J.-H., Wu, Y.-N., & Wang, M.-L. (2019). Determination of 107 Pesticide Residues in Wolfberry with Acetate-buffered Salt Extraction and Sin-QuEChERS Nano Column Purification Coupled with Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Molecules, 24(16), 2918. https://doi.org/10.3390/molecules24162918




