Rosmarinic Acid, the Main Effective Constituent of Orthosiphon stamineus, Inhibits Intestinal Epithelial Apoptosis Via Regulation of the Nrf2 Pathway in Mice
Abstract
:1. Introduction
2. Results
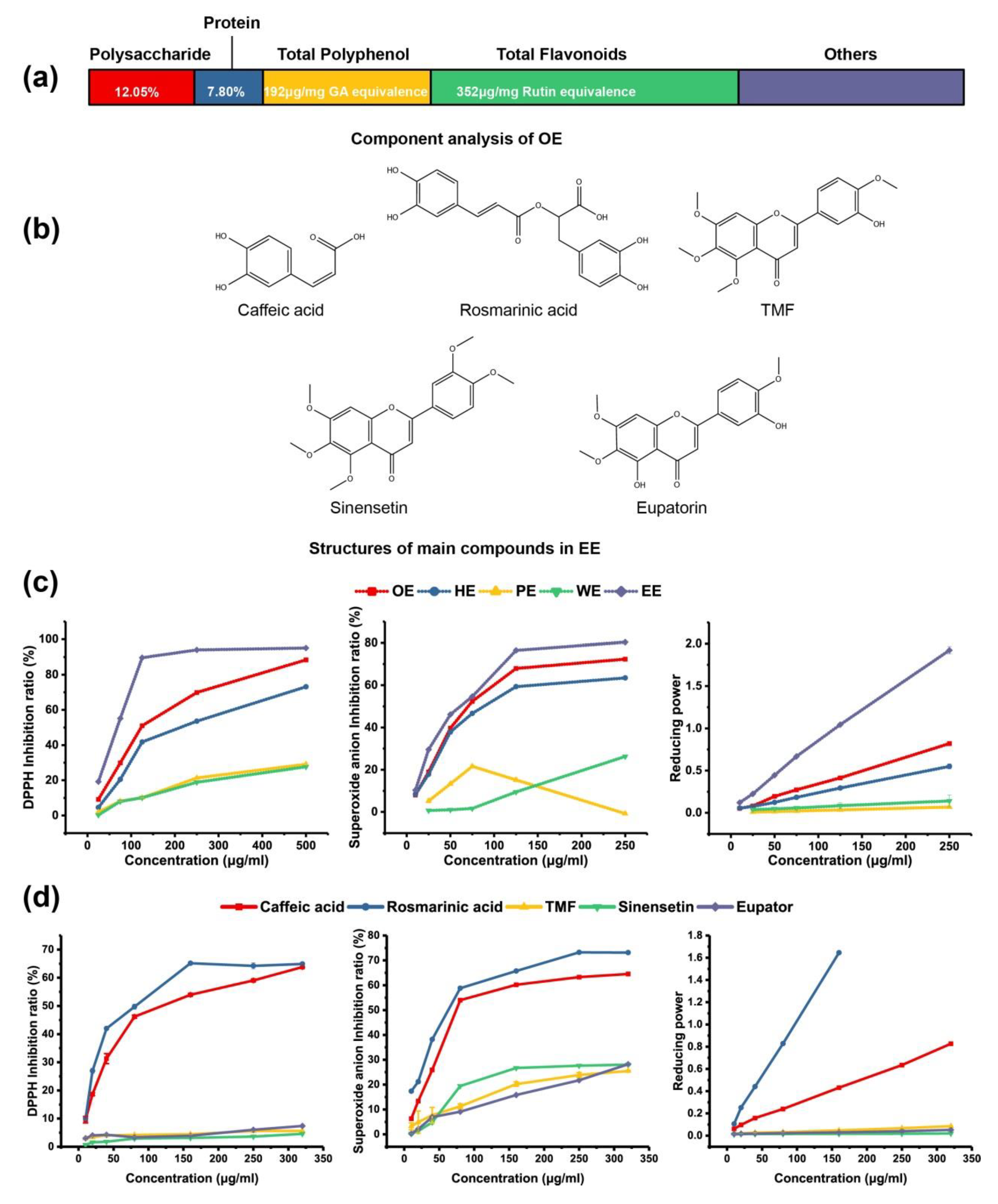
2.1. RA is the Main Antioxidant in OE
2.2. OE and RA Showed Antioxidant Activity in vivo But Did Not Alter Body Weight
2.3. OE and RA Antioxidant Activity via the Nrf2 Pathway
2.4. OE and RA Alter the Ratio of Villus and Crypt Structure in Mice
2.5. OE and RA Inhibit Apoptosis of Intestinal Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials
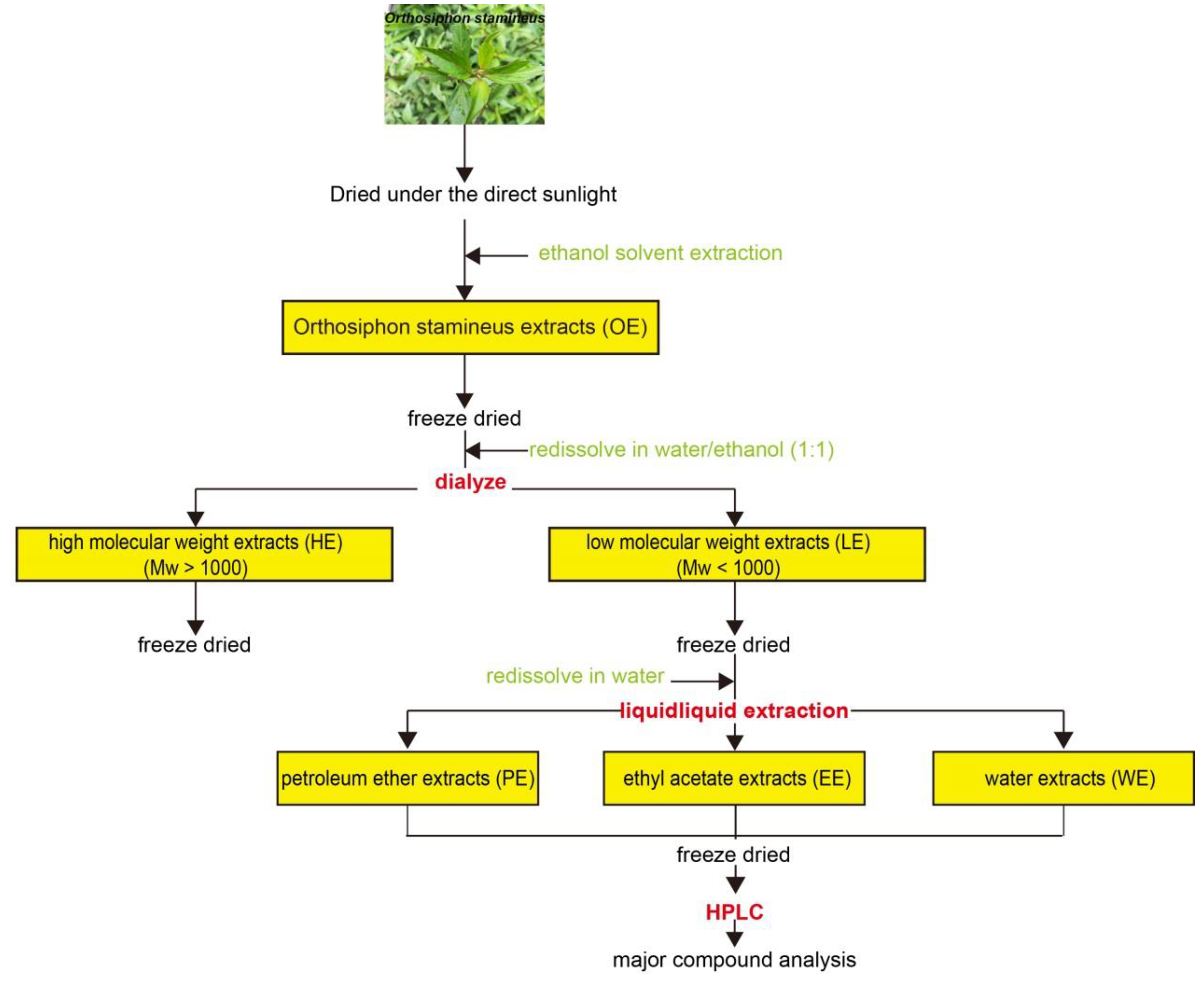
4.2. Solvent Extraction and Partition Fractionation
4.3. Composition of OE
4.4. Reducing Power and Free Radical Scavenging Activity Analysis
4.5. Animals and Treatment
4.6. Measurement of Oxidative Stress in Mice
4.7. Quantitative Real-Time PCR Analysis
4.8. EMSA
4.9. Measurement of Villus Height and Crypt Depth
4.10. DNA Fragmentation Analysis
4.11. Analysis of Caspase-3, -8, and -9 Activity
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erdelyi, I.; Levenkova, N.; Lin, E.Y.; Pinto, J.T.; Lipkin, M.; Quimby, F.W.; Holt, P.R. Western-Style Diets Induce Oxidative Stress and Dysregulate Immune Responses in the Colon in a Mouse Model of Sporadic Colon Cancer. J. Nutr. 2009, 139, 2072–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.S.; Yu, C.H.; Ying, H.Z.; Wang, Z.Y.; Cai, H.F. Prophylactic effects of Orthosiphon stamineus Benth. extracts on experimental induction of calcium oxalate nephrolithiasis in rats. J. Ethnopharmacol. 2012, 144, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Ameer, O.Z.; Salman, I.M.; Asmawi, M.Z.; Ibraheem, Z.O.; Yam, M.F. Orthosiphon stamineus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. J. Med. Food 2012, 15, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Sumaryono, W.; Proksch, P.; Wray, V.; Witte, L.; Hartmann, T. Qualitative and Quantitative Analysis of the Phenolic Constituents from Orthosiphon aristatus. Planta Med. 1991, 57, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, H.-R.; Woo, E.-R.; Hong, S.-T.; Chae, H.-J.; Chae, S.-W. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem. Pharmacol. 2005, 70, 1066–1078. [Google Scholar] [CrossRef]
- Cai, X.; Xiao, C.; Xue, H.; Xiong, H.; Hang, Y.; Xu, J.; Lu, Y. A comparative study of the antioxidant and intestinal protective effects of extracts from different parts of Java tea (Orthosiphon stamineus). Food Sci. Nutr. 2018, 6, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Buttke, T.M.; Sandstrom, P.A. Oxidative stress as a mediator of apoptosis. Immunol. Today 1994, 15, 7–10. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liang, X.; Xie, Y. Qualitative and quantitative analysis on the chemical constituents in Orthosiphon stamineus Benth. using ultra high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed Anal. 2018, 164, 135–147. [Google Scholar] [CrossRef]
- Matkowski, A. Antioxidant Activity of Extracts and Different Solvent Fractions of Glechoma hederacea L. and Orthosiphon stamineus (Benth.). Kudo. Adv. Clin. Exp. Med. 2008, 17, 615–624. [Google Scholar]
- Palafox-Carlos, H.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, A.G. Antioxidant Interactions between Major Phenolic Compounds Found in ‘Ataulfo’ Mango Pulp: Chlorogenic, Gallic, Protocatechuic and Vanillic Acids. Molecules 2012, 17. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Sun, J.; Qiao, Y.; Qi, C.; Jiang, W.; Xiao, H.; Shi, Y.; Le, G.-W. High-fat-diet–induced obesity is associated with decreased antiinflammatory Lactobacillus reuteri sensitive to oxidative stress in mouse Peyer’s patches. Nutrition 2016, 32, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Redox biology of the intestine. Free Radic. Res. 2011, 45, 1245–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ausman, L.M.; Russell, R.M.; Greenberg, A.S.; Wang, X.-D. Increased Apoptosis in High-Fat Diet–Induced Nonalcoholic Steatohepatitis in Rats Is Associated with c-Jun NH2-Terminal Kinase Activation and Elevated Proapoptotic Bax. J. Nutr. 2008, 138, 1866–1871. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, L.; Chen, X.; Sheng, Y.; Guo, Q.; Bao, J.; Xu, J. X/XO or H2O2 induced IPEC-J2 cell as a new in vitro model for studying apoptosis in post-weaning piglets. Cytotechnology 2016, 68, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Ulukaya, E.; Acilan, C.; Yilmaz, Y. Apoptosis: Why and how does it occur in biology? Cell Biochem. Funct. 2011, 29, 468–480. [Google Scholar] [CrossRef]
- Gao, L.P.; Gao, L.P.; Wei, H.L.; Zhao, H.S.; Xiao, S.Y.; Zheng, R.L. Antiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Die Pharm. - Int. J. Pharm. Sci. 2005, 60, 62–65. [Google Scholar]
- Domitrović, R.; Potočnjak, I.; Crnčević-Orlić, Ž.; Škoda, M. Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem. Toxicol. 2014, 66, 321–328. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, B.J.; Kim, J.H.; Yu, Y.S.; Kim, M.Y.; Kim, K.-W. Rosmarinic acid suppresses retinal neovascularization via cell cycle arrest with increase of p21WAF1 expression. Eur. J. Pharmacol. 2009, 615, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Encalada, M.A.; Hoyos, K.M.; Rehecho, S.; Berasategi, I.; de Ciriano, M.G.-Í.; Ansorena, D.; Astiasarán, I.; Navarro-Blasco, Í.; Cavero, R.Y.; Calvo, M.I. Anti-proliferative Effect of Melissa officinalis on Human Colon Cancer Cell Line. Plant Foods Hum. Nutr. 2011, 66, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Iswandana, R.; Pham, B.T.; van Haaften, W.T.; Luangmonkong, T.; Oosterhuis, D.; Mutsaers, H.A.M.; Olinga, P. Organ- and species-specific biological activity of rosmarinic acid. Toxicol. Vitr. 2016, 32, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Stępkowski, T.M.; Kruszewski, M.K. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic. Biol. Med. 2011, 50, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, X.J. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J. Gastroenterol. 2016, 22, 8905–8909. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Shaari, A.R.; Azimi, A. Effect of Drying Methods on Metabolites Composition of Misai Kucing (Orthosiphon stamineus) Leaves. In 3rd International Conference on Biotechnology and Food Science; Dan, Y., Ed.; Elsevier B.V.: Bangkok, Thailand, 2012; Volume 2, pp. 178–182. [Google Scholar]
- Chueh, W.H.; Lin, J.Y. Berberine, an isoquinoline alkaloid, inhibits streptozotocin-induced apoptosis in mouse pancreatic islets through down-regulating Bax/Bcl-2 gene expression ratio. Food Chem. 2011, 132, 252–260. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: not available. |



| Items | NC | FC | OE | RA |
|---|---|---|---|---|
| GSH (μmol/mg prot) | 5.85 ± 0.99a | 2.70 ± 1.09b | 4.54 ± 0.88ab | 4.81 ± 0.81ab |
| GSH/GSSG | 3.79 ± 0.23 | 3.16 ± 0.39 | 3.57 ± 0.32 | 3.48 ± 0.51 |
| MDA (nmol/mg prot) | 2.82 ± 0.26c | 6.38 ± 0.66a | 4.16 ± 0.58bc | 4.60 ± 0.39b |
| 8-OH-dG (pg/mg prot) | 72.43 ± 12.94b | 294.21 ± 27.22a | 121.54 ± 27.91b | 171.17 ± 42.55b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Yang, F.; Zhu, L.; Xia, Y.; Wu, Q.; Xue, H.; Lu, Y. Rosmarinic Acid, the Main Effective Constituent of Orthosiphon stamineus, Inhibits Intestinal Epithelial Apoptosis Via Regulation of the Nrf2 Pathway in Mice. Molecules 2019, 24, 3027. https://doi.org/10.3390/molecules24173027
Cai X, Yang F, Zhu L, Xia Y, Wu Q, Xue H, Lu Y. Rosmarinic Acid, the Main Effective Constituent of Orthosiphon stamineus, Inhibits Intestinal Epithelial Apoptosis Via Regulation of the Nrf2 Pathway in Mice. Molecules. 2019; 24(17):3027. https://doi.org/10.3390/molecules24173027
Chicago/Turabian StyleCai, Xuan, Fan Yang, Lihui Zhu, Ye Xia, Qingyuan Wu, Huiqin Xue, and Yonghong Lu. 2019. "Rosmarinic Acid, the Main Effective Constituent of Orthosiphon stamineus, Inhibits Intestinal Epithelial Apoptosis Via Regulation of the Nrf2 Pathway in Mice" Molecules 24, no. 17: 3027. https://doi.org/10.3390/molecules24173027






