Microwave-Assisted Extraction Combined with In-Capillary [Fe(ferrozine)3]2+-CE-DAD to Screen Active Components with the Ability to Chelate Ferrous Ions from Flos Sophorae Immaturus (Flos Sophorae)
Abstract
:1. Introduction
2. Result and Discussion
2.1. Optimization of Microwave-Assisted Extraction Parameters
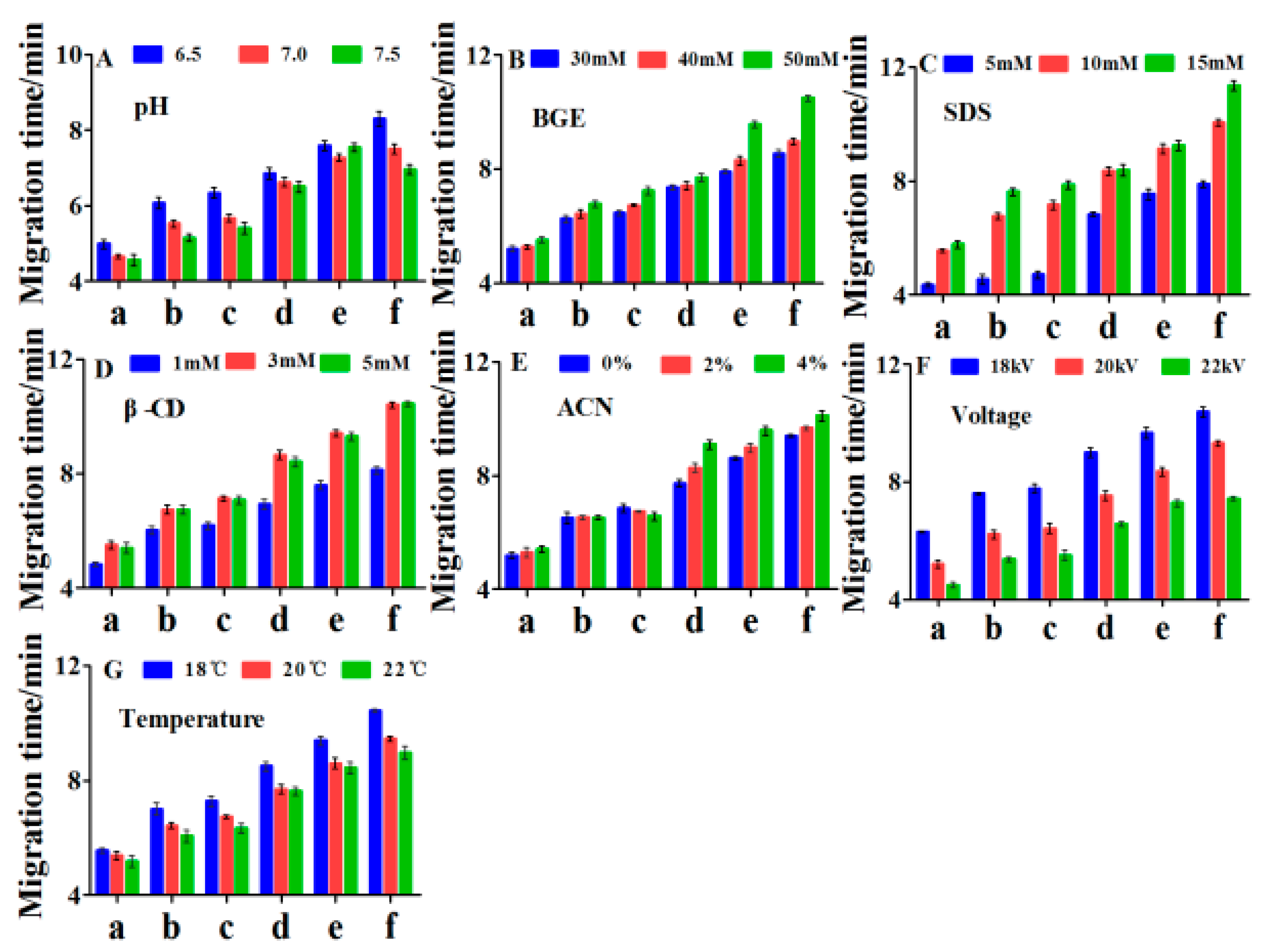
2.2. Optimization of On-Line Method
2.2.1. Effect of Buffer pH
2.2.2. Effect of Buffer Concentration
2.2.3. Effect of SDS Concentration
2.2.4. Effect of β-CD (β-cyclodextrin) Concentration
2.2.5. Effect of Acetonitrile Concentration
2.2.6. Effect of Voltage and Temperature
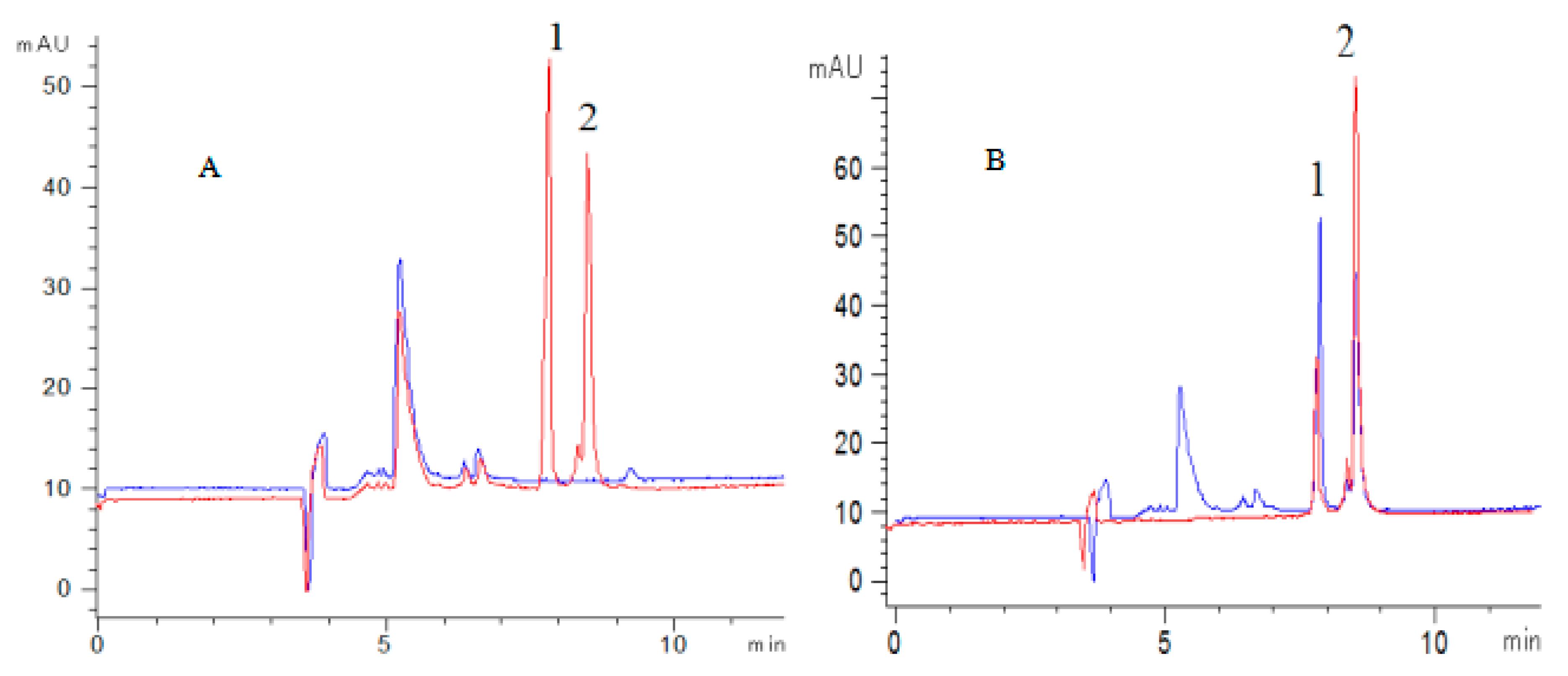
2.3. Method Validation
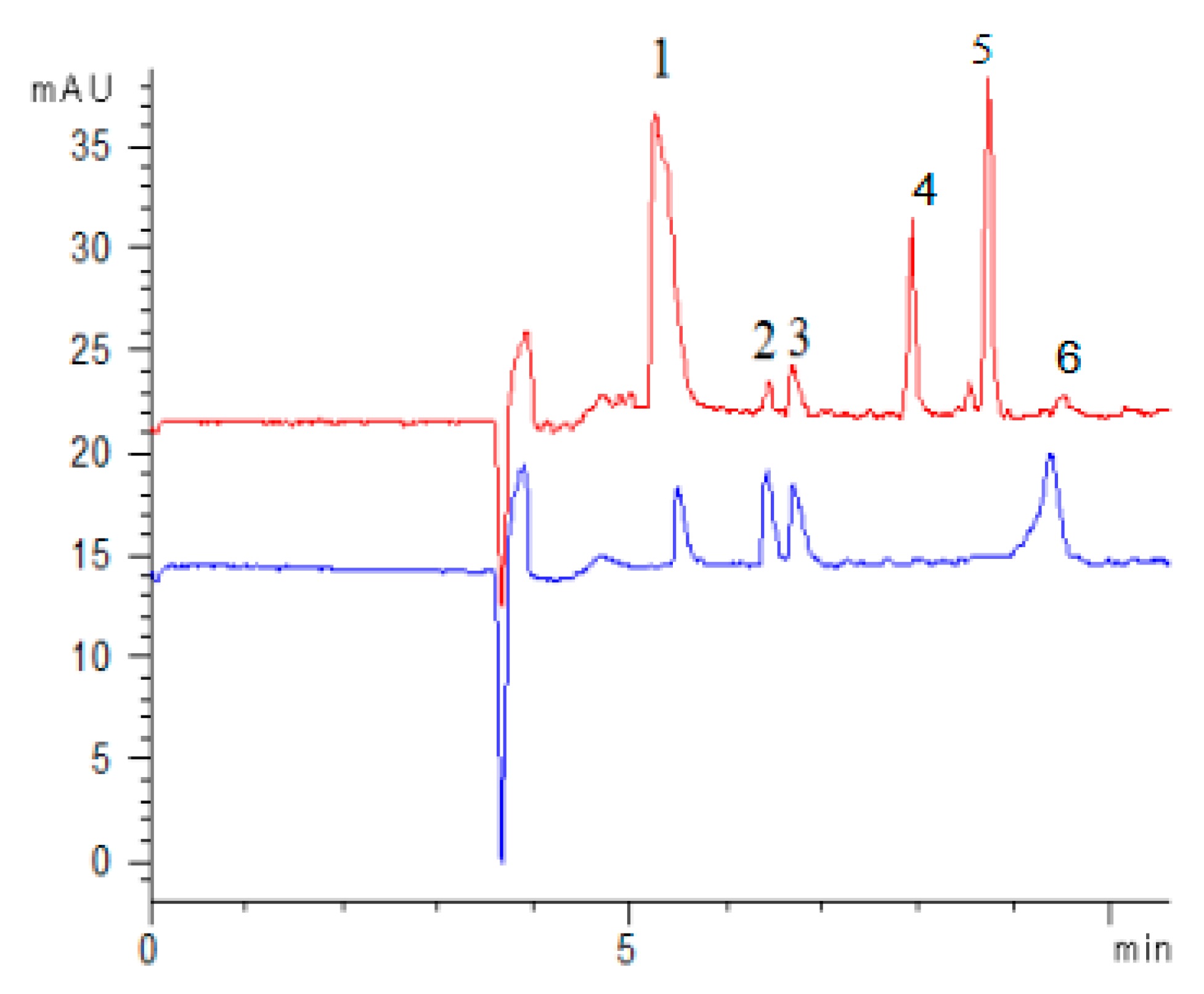
2.4. Method Application
2.4.1. Screening of Antioxidants of FSI(FS)
2.4.2. Determination of the Activity and Contents of the Herbal Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Analytical Samples
3.3. Instruments and CE Condition
3.4. The Conditions and Principle of Method
3.5. Method Validation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grigalius, I.; Petrikaite, V. Relationhip between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Karan, M.; Dogra, R. Ameliorating effect of Celastrus paniculatus standardized extract and its fraction on 3-nitropropionsic acid induced neuronal damage in rats: Possible antioxidant mechanism. Pharm. Biol. 2017, 55, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhang, J.H.; Cao, J.B.; Wang, W.; Wang, D.X.; Zhang, X.Y.; Yu, J.; Zhang, Y.Y.; Zhang, Y.Z.; Mi, W.D. Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J. Neuroinflamm. 2017, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Gerzson, M.F.B.; Victoria, F.N.; Radatz, C.S.; De Gomes, M.G.; De Castro, B.B. In Vitro Antioxidant Activity and in Vivo Antidepressant-like Effect of α-(phenylselanyl) Acetophenone in Mice. Pharmacol. Biochem. Behav. 2012, 102, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, J.; Rodrigues, A.F.; De Sousa Rós, A. Ferulic acid chronic treatment exerts antidepressant-like effect: Role of antioxidant defense system. Metab. Brain Dis. 2015, 30, 1453–1463. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.A.; Galar-Martínez, M.; García-Rodríguez, R.V.; Chamorro-Cevallos, G.A.; Herna’ndez-Reyes, A.G. Antioxidant Effect of Spirulina(Arthrospira) maxima on Chronic Inflammation Induced by Freund’s Complete Adjuvant in Rats. J. Med. Food 2015, 18, 865–871. [Google Scholar] [CrossRef]
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Mahmood, A. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharm. Biol. 2016, 54, 1971–1981. [Google Scholar] [CrossRef]
- Cui, S.C.; Yu, J.; Zhang, X.H.; Cheng, M.Z.; Yang, L.W.; Xu, J.Y. Antihyperglycemic and antioxidant activity of water extract from Anoectochilus roxburghii in experimental diabetes. Exp. Toxicol. Pathol. 2013, 65, 485–488. [Google Scholar] [CrossRef]
- Cho, E.; Choi, J.M.; Kim, H.; Tahir, M.N.; Choi, Y.; Jung, S. Ferrous iron chelating property of low-molecular weight succinoglycans isolated fromSinorhizobium meliloti. BioMetals 2013, 26, 321–328. [Google Scholar] [CrossRef]
- Victoria, F.N.; Anversa, R.; Penteado, F.; Castro, M.; Lenardão, E.J.; Savegnago, L. Antioxidant and antidepressant-like activities of semi-synthetic α-phenylseleno citronellal. Eur. J. Pharmacol. 2014, 742, 131–138. [Google Scholar] [CrossRef]
- Panigrahi, J.; Patel, M.; Patel, N.; Gheewala, B.; Gantait, S. Changes in antioxidant and biochemical activities in castor oil-coated Capsicum annuum L. during postharvest storage. 3 Biotech 2018, 8, 280–288. [Google Scholar] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kucˇa, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.K.B.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Et Biophys. Acta 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Li, D.; Ma, X.K.; Jiang, F.Q.; He, Q.; Qiu, S.L.; Li, Y.; Wang, G. Optimization of Ionic Liquid-Assisted Extraction of Biflavonoids from Selaginella doederleinii and Evaluation of Its Antioxidant and Antitumor Activity. Molecules 2017, 22, 586. [Google Scholar]
- Li, B.; Zhang, N.; Wang, D.X.; Jiao, L.L.; Tan, Y.; Wang, J.; Li, H.; Wu, W.; Jiang, D.C. Structural analysis and antioxidant activities of neutral polysaccharide isolated from Epimedium koreanum Nakai. Carbohydr. Polym. 2018, 196, 246. [Google Scholar] [CrossRef] [PubMed]
- Butkute, B.; Dagilyte, A.; Benetis, R.; Padarauskas, A.; Ceseviciene, J.; Olšauskaite, V.; Lemeziene, N. Mineral and Phytochemical Profiles and Antioxidant Activity of Herbal Material from Two Temperate Astragalus Species. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Pravadali-Cekic, S.; Dennis, G.R.; Bashir, R.; Mahon, P.J.; Shalliker, R.A. Ferric reducing antioxidant potential(FRAP) of antioxidants using reaction flow chromatography. Anal. Chim. Acta 2017, 967, 93–101. [Google Scholar] [CrossRef]
- Wang, W.F.; Yang, J.L. Advances in screening enzyme inhibitors by capillary electrophoresis. Electrophoresis 2019, 40, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- María, R.P.; Juan, A.O.G.; Rut, M.F.T.; Andreu, L.; Miguel Ángel, B.L. Recent trends in capillary electrophoresis for complex samples analysis: A review. Electrophoresis 2018, 39, 111–125. [Google Scholar]
- Wuethrich, A.; Quirino, J.P. Derivatisation for separation and detection in capillary electrophoresis (2015–2017). Electrophoresis 2018, 39, 82–96. [Google Scholar] [CrossRef]
- Ma, H.F.; Li, J.; An, R.M.; Gao, X.M.; Chang, Y.X. A powerful on line ABTS+–CE-DAD method to screen and quantify major antioxidants for quality control of Shuxuening Injection. Sci. Rep. 2018, 8, 5441. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.X.; Liu, J.; Bai, Y.; Li, J.; Liu, E.W.; He, J.; Jiao, X.C.; Wang, Z.Z.; Gao, X.M.; Zhang, B.L. The Activity-Integrated Method for Quality Assessment of Reduning Injection by On-Line DPPH-CE-DAD. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Liu, W.; Yu, X.A.; Cao, J.; An, M.R.; Gao, X.M.; Chang, Y.X. A Green Antioxidant Activity-Integrated Dual-Standard Method for Rapid Evaluation of the Quality of Traditional Chinese Medicine Xuebijing Injection by On-Line DPPH-CE-DAD. Evid. Based Complement. Altern. Med. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.R.; Bai, Y.J.; Zhao, Z.F.; Wang, X.X.; Fang, J.C.; Huang, L.H.; Zeng, M.; Zhang, Q.; Zhang, Y.J.; Zheng, X.H. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L. A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J. Antioxidant and Anti-inflammatory Activities of Selected Medicinal Plants Containing Phenolic and Flavonoid components. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.R.; Wang, S.T.; Chen, Y.; Wang, H.Y. Composition and Antibacterial Activity of Essential Oils of Flos Sophorae Immaturus. Int. J. Food Prop. 2011, 14, 903–913. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.R.; Li, L.Y.; Tan, J.; Song, X.H.; Chen, D.X.; Xu, J.; Ding, G. Variation in the Components and Antioxidant and Tyrosinase Inhibitory Activities of Styphnolobium Japonicum(l.) Schott Extract During Flower Maturity Stages. Chem. Biodivers. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhang, H.Y.; Chen, G. Carbon nanotube/polystyrene composite electrode for microchip electrophoretic determination of rutin and quercetin in flos sophorae immaturus. Talanta 2007, 73, 932–937. [Google Scholar] [CrossRef]
- Xie, Z.S.; Sun, Y.J.; Lam, S.C.; Zhao, M.Q.; Liang, Z.K.; Yu, X.X.; Yang, D.P.; Xu, X.J. Extraction and isolation of flavonoid glycosides from Flos Sophorae Immaturus using ultrasonic-assisted extraction followed by high-speed countercurrent chromatography. J. Sep. Sci. 2014, 37, 957–965. [Google Scholar] [CrossRef]
- Gan, Z.B.; Chen, Q.W.; Fu, Y.J.; Chen, G. Determination of bioactive constituents in Flos Sophorae Immaturus and Cortex Fraxini by capillary electrophoresis in combination with far infrared-assisted solvent extraction. Food Chem. 2012, 130, 1122–1126. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Wang, S.B.; Yan, H.W.; Liao, K.J.; Dai, Y. Microwave-assisted extraction of rutin from glutinous rice. Chem. Technol. 2009, 17, 25–26. [Google Scholar]
- Wu, H.W.; Chen, M.L.; Fan, Y.C.; Elsebaei, F.; Zhu, Y. Determination of rutin and quercetin in Chinese herbal medicine by ionsic liquid-based pressurized liquid extraction–liquid chromatography–chemiluminescence detection. Talanta 2012, 88, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extraction of active components from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Liu, Y.H.; Yang, C.L.; Liu, L.H. On-line double focusing of atenolol and metoprolol in human urine using capillary electrophoresis with the aid of β-cyclodextrin. Anal. Chim. Acta 2017, 978, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Davis, I.; Liu, A.; Miller, A.; Shamsi, S.A. Improved separation and detection of picolinic acid and quinolinic acid by capillary electrophoresis-mass spectrometry: Application to analysis of human cerebrospinal fluid. J. Chromatogr. A 2013, 1316, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Brusseau, M.L.; Zang, X.; Lu, S.G.; Zhang, X.; Farooq, U.; Qiu, Z.F.; Sui, Q. Enhanced effect of HAH on citric acid-chelated Fe(II)-catalyzed percarbonate for trichloroethene degradation. Environ. Sci. Pollut. Res. 2017, 24, 24318–24326. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Pegg, R.B. Limitation of the Tetramethylmurexide Assay for Investigating the Fe(II) Chelation Activity of Phenolic components. J. Agric. Food Chem. 2009, 57, 6425–6431. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Willmore, W.G.; Tsopmo, A. Peptidomic analysis of hydrolyzed oat bran proteins, and their in vitro antioxidant and metal chelating properties. Food Chem. 2019, 279, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sci. 2005, 76, 863–875. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrieres, J.; Cerf, D.L.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
Sample Availability: Samples of Rutin, Kaempferol-3-rutinoside, Narcissoside, Quercetin are available from the authors. |


| Factors | Levels | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| (A) Methanol concentration (%) | 50 | 75 | 100 |
| (B) Microwave time (min) | 2.5 | 5 | 7.5 |
| (C) Microwave power (W) | 250 | 500 | 750 |
| (D) Solid/liquid ratio (g∙mL−1) | 1:10 | 1:20 | 1:50 |
| No. | Factors | Inhibition Rates (%) | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 1 | 1 | 1 | 1 | 31.12 |
| 2 | 1 | 2 | 2 | 2 | 40.28 |
| 3 | 1 | 3 | 3 | 3 | 52.69 |
| 4 | 2 | 1 | 2 | 3 | 31.02 |
| 5 | 2 | 2 | 3 | 1 | 46.16 |
| 6 | 2 | 3 | 1 | 2 | 34.48 |
| 7 | 3 | 1 | 3 | 2 | 28.81 |
| 8 | 3 | 2 | 1 | 3 | 15.73 |
| 9 | 3 | 3 | 2 | 1 | 26.4 |
| K1j a | 41.363 | 30.317 | 27.110 | 34.560 | |
| K2j | 37.220 | 34.057 | 32.567 | 34.523 | |
| K3j | 23.647 | 37.857 | 42.553 | 33.147 | |
| Rib | 17.716 | 7.540 | 15.443 | 1.413 | |
| O c | A1 | B3 | C3 | D1 | |
| Source | Sum of Squares | Degrees of Freedom | F-Value | p-Value |
|---|---|---|---|---|
| A | 515.283 | 2 | 132.327 | * |
| B | 85.279 | 2 | 21.900 | * |
| C | 368.005 | 2 | 94.506 | * |
| D | 3.894 | 2 | 1 | |
| Error | 3.89 |
| Compounds | Regression Equation | r | Linearity Range (μg·mL−1) | LOD (μg·mL−1) | LOQ (μg·mL−1) | Recovery | |
|---|---|---|---|---|---|---|---|
| Average (%) | RSD (%) | ||||||
| Rutin | Y = 0.2922x − 0.2637 | 0.9999 | 62.5–2000 | 1.05 | 3.50 | 104 | 4.33 |
| Kaempferol- 3-rutinoside | Y = 0.2882x + 0.225 | 0.9988 | 3.125–100 | 0.9 | 3 | 96.3 | 1.10 |
| Narcissoside | Y = 0.3255x − 0.5841 | 0.9978 | 5–160 | 1.05 | 3.50 | 102 | 4.63 |
| Quercetin | Y = 0.8004x − 0.6256 | 0.9995 | 3.125–100 | 0.75 | 2.50 | 95.2 | 5.10 |
| Compounds | Ca (µg∙mL−1) | Intraday | Interday | Stability for 24 h | |||
|---|---|---|---|---|---|---|---|
| Accuracy (%) | RSD (%) | Accuracy (%) | RSD (%) | Remains (%) | RSD (%) | ||
| Rutin | 250 | 98 | 2 | 99.4 | 1.75 | 102 | 1.01 |
| 500 | 101 | 0.873 | 100 | 0.853 | 102 | 3.51 | |
| 1000 | 100 | 0.299 | 99.2 | 1.39 | 101 | 0.73 | |
| Kaempferol-3-rutinoside | 12.5 | 101 | 1.99 | 101 | 2.30 | 104 | 1.91 |
| 25 | 103 | 2.98 | 102 | 2.39 | 101 | 2.37 | |
| 50 | 101 | 1 | 100 | 1.03 | 102 | 3 | |
| Narcissoside | 20 | 104 | 0.951 | 104 | 2.32 | 103 | 2.32 |
| 40 | 102 | 1.14 | 101 | 1.27 | 103 | 1.49 | |
| 80 | 101 | 0.580 | 101 | 0.735 | 101 | 2.09 | |
| Quercetin | 12.5 | 102 | 1.56 | 101 | 1.63 | 100 | 1.65 |
| 25 | 101 | 1.78 | 100 | 1.22 | 100 | 1.54 | |
| 50 | 99.9 | 0.830 | 100 | 1.15 | 100 | 1.44 | |
| [Fe(ferrozine)3]2+ | − | − | 0.768 | − | 0.580 | − | 0.908 |
| Samples | Flos Sophorae Immaturus | Flos Sophorae | Citric Acid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HeNan | HeBei | GuangXi | ShanDong | ShanXi | HeBei | HeNan | ShanDong | ||
| Rutin | 210.46 ± 3.49 | 208.81 ± 1.64 | 211.48 ± 0.79 | 188.11 ± 5.71 | 164.91 ± 5.24 | 78.09 ± 1.13 | 79.45 ± 1.11 | 76.91 ± 2.66 | - |
| Kaempferol-3-rutinoside | 10.73 ± 0.48 | 10.51 ± 0.66 | 14.65 ± 1.31 | 7.78 ± 0.6 | 11.36 ± 0.69 | 6.98 ± 0.66 | 5.65 ± 0.16 | 6.8 ± 0.53 | - |
| Narcissoside | 6.69 ± 0.32 | 3.9 ± 0.39 | 8.22 ± 0.8 | 6.41 ± 0.46 | 4.83 ± 0.54 | 2.24 ± 0.29 | 2.31 ± 0.29 | 2.01 ± 0.06 | - |
| Quercetin | 4.88 ± 0.46 | 7.76 ± 0.28 | 8.1 ± 0.32 | 6.37 ± 0.46 | 8.76 ± 0.74 | 7.36 ± 0.34 | 8.23 ± 0.59 | 9.17 ± 0.42 | - |
| IC50 (mg/mL) | 18.41 | 17.73 | 17.36 | 17.11 | 20.25 | 24.67 | 22.78 | 25.44 | 3.681 |
| R2 | 0.991 | 0.9952 | 0.9778 | 0.9834 | 0.9880 | 0.9984 | 0.9937 | 0.9925 | 0.9845 |
| Extraction Method | Extraction Time (min) | Solvent | Yield of Rutin (mg/g) | Yield of Quercetin (mg/g) | Reference |
|---|---|---|---|---|---|
| Heating reflux | 180 | 100% MeOH | 164.4–211 | 4.86–5.40 | [28] |
| UAE | 60 | 82% MeOH | 208.6 | − | [29] |
| FIASE | 6 | 100% MeOH | 202 | 8.033 | [30] |
| MAE | 4 | 65% EtOH | 208.6 | − | [31] |
| IL-PLE | 5 | Ionic liquid | 196.3 | 5.18 | [32] |
| MAE | 7.5 | 50% MeOH | 211.48 | 8.36 | Adapted in this study |
| Samples | Chelating Agents | Solvent Volume (mL) | Reaction Time (min) | Extraction Solvent | Detection | Screening of Antioxidants | Reference |
|---|---|---|---|---|---|---|---|
| Phenolic compounds | Tetramethylmurexide | 2.1 | 10 | 80% acetone | spectrophotometer | × | [37] |
| protein hydrolysates | Ferrozine | 1.7 | 30 | deionized water | spectrophotometer | × | [38] |
| Echinochrome | EDTA | − | 10 | 96% ethanol | spectrophotometer | × | [39] |
| Water-Soluble polysaccharides | Ferrozine | 0.7 | 15 | 96% ethanol | spectrophotometer | × | [40] |
| FSI(FS) | Ferrozine | 0.084 | 5 | 50% methanol | Capillary electrophoresis | √ | Proposed in this study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wang, S.; Ma, H.; Jin, H.; Li, J.; Yang, X.; Gao, X.; Chang, Y. Microwave-Assisted Extraction Combined with In-Capillary [Fe(ferrozine)3]2+-CE-DAD to Screen Active Components with the Ability to Chelate Ferrous Ions from Flos Sophorae Immaturus (Flos Sophorae). Molecules 2019, 24, 3052. https://doi.org/10.3390/molecules24173052
Liu T, Wang S, Ma H, Jin H, Li J, Yang X, Gao X, Chang Y. Microwave-Assisted Extraction Combined with In-Capillary [Fe(ferrozine)3]2+-CE-DAD to Screen Active Components with the Ability to Chelate Ferrous Ions from Flos Sophorae Immaturus (Flos Sophorae). Molecules. 2019; 24(17):3052. https://doi.org/10.3390/molecules24173052
Chicago/Turabian StyleLiu, Tao, Shanshan Wang, Huifen Ma, Hua Jin, Jin Li, Xuejing Yang, Xiumei Gao, and Yanxu Chang. 2019. "Microwave-Assisted Extraction Combined with In-Capillary [Fe(ferrozine)3]2+-CE-DAD to Screen Active Components with the Ability to Chelate Ferrous Ions from Flos Sophorae Immaturus (Flos Sophorae)" Molecules 24, no. 17: 3052. https://doi.org/10.3390/molecules24173052
APA StyleLiu, T., Wang, S., Ma, H., Jin, H., Li, J., Yang, X., Gao, X., & Chang, Y. (2019). Microwave-Assisted Extraction Combined with In-Capillary [Fe(ferrozine)3]2+-CE-DAD to Screen Active Components with the Ability to Chelate Ferrous Ions from Flos Sophorae Immaturus (Flos Sophorae). Molecules, 24(17), 3052. https://doi.org/10.3390/molecules24173052





