Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation during Msalais Winemaking
Abstract
1. Introduction
2. Results
2.1. Furaneol Content of Msalais
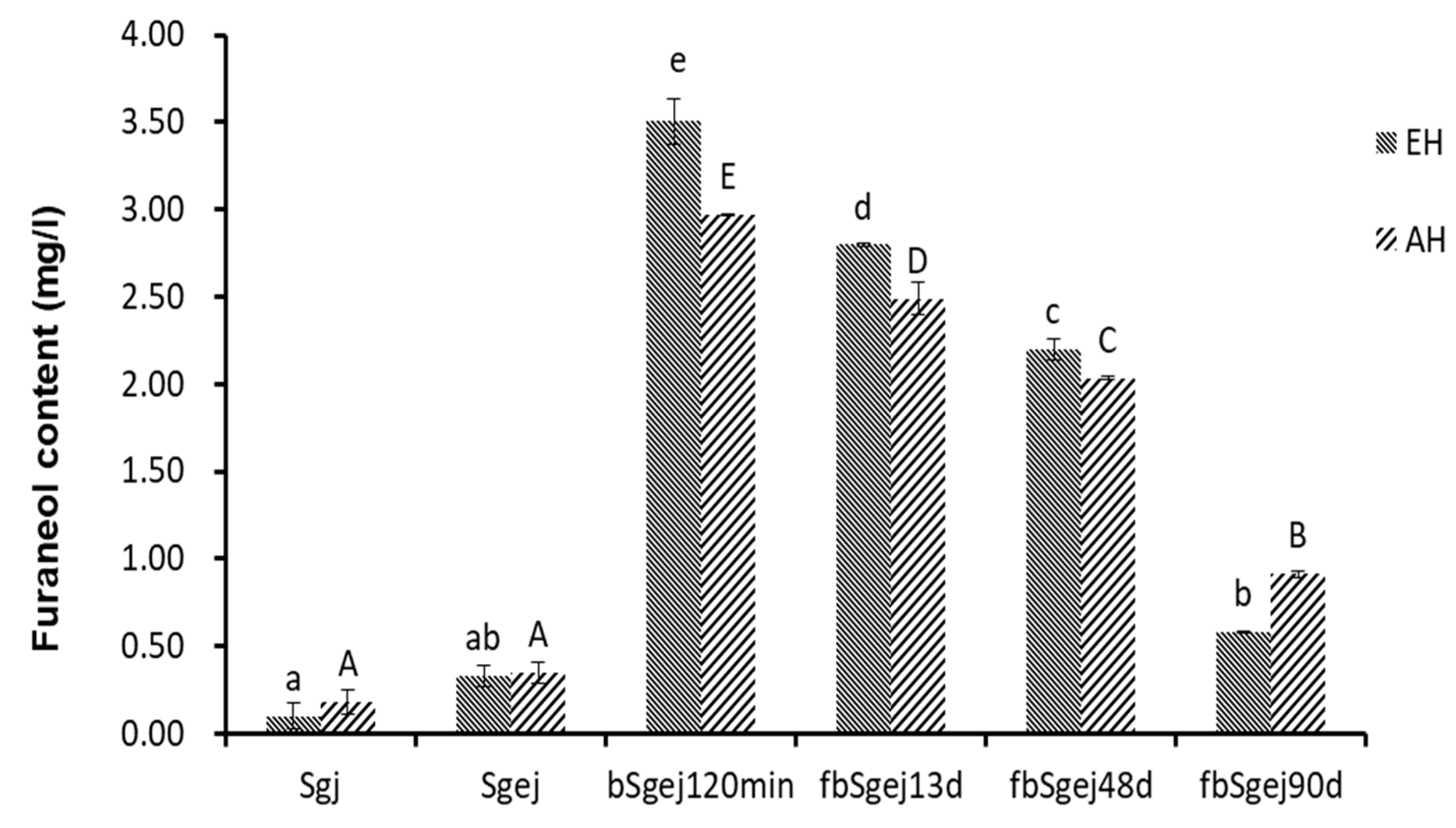
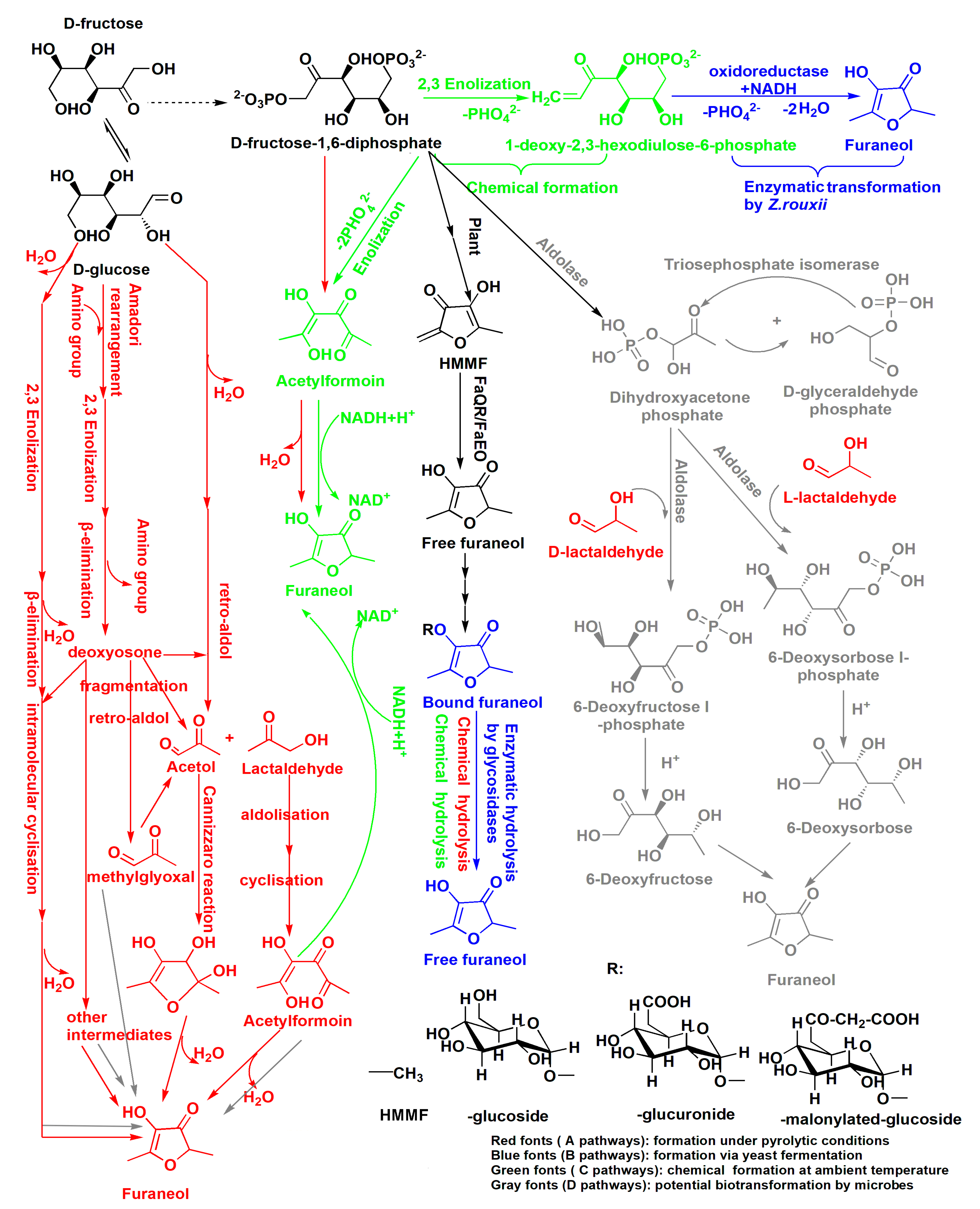
2.2. Formation of Furaneol during Msalais-Making
2.3. Analysis of Furaneol Released by Hydrolysis from Furaneol Glucosides
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Msalais
4.3. Preparation of Models for Simulated Msalais-Making
4.4. Enzymatic and Thermal Acidic Hydrolysis of Furaneol Glucosides
4.5. HPLC Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hodge, J. Novel Reductones and Methods of Making Them. U.S. Patent 2,936,308, 10 May 1960. [Google Scholar]
- Guedes de Pinho, P.; Bertrand, A. Analytical determination of furaneol (2,5-dimethyl1-4-hydroxy-3(2H)-furanone). Application to differentiation of white wines from hybrid and various Vitis vinifera cultivars. Am. J. Enol. Vitic. 1995, 46, 181–186. [Google Scholar]
- Rodin, J.O.; Himel, C.M.; Silverstein, R.M.; Lrrprt, R.W.; Gortner, W.A. Volatile Flavor and Aroma Components of Pineapple. l. Isolation and Tentative Identification of 2,5-Dimethyl-4-Hydroxy-3(2H)-Furanone. J. Food Sci. 1965, 30, 280–285. [Google Scholar] [CrossRef]
- Sasaki, K.; Takase, H.; Tanzawa, F.; Kobayashi, H.; Saito, H.; Matsuo, H.; Takata, R. Identification of furaneol glucopyranoside, the precursor of strawberry-like aroma, furaneol, in Muscat Bailey A. Am. J. Enol. Vitic. 2015, 66, 91–94. [Google Scholar] [CrossRef]
- Baek, H.H.; Cadwallader, K.R. Contribution of free and glycosidically bound volatile compounds to the aroma of muscadine grape juice. J. Food Sci. 1999, 64, 441–444. [Google Scholar] [CrossRef]
- Buttery, R.G.; Takeoka, G.R.; Ling, L.C. Furaneol: Odor Threshold and Importance to Tomato Aroma. J. Agric. Food Chem. 1995, 43, 1638–1640. [Google Scholar] [CrossRef]
- Krammer, G.E.; Takeoka, G.R.; Buttery, R.G. Isolation and Identification of 2,5-Dimethyl-4-hydroxy-3(2H)-furanone glucoside from tomatoes. J. Agric. Food Chem. 1994, 42, 1595–1597. [Google Scholar] [CrossRef]
- Maga, J.A.; Katz, I. Furans in foods. CRC Crit. Rev. Food Sci. Nutr. 1979, 11, 355–400. [Google Scholar] [CrossRef] [PubMed]
- Mutti, B.; Grosch, W. Potent odorants of boiled potatoes. Manag. Des. 2004, 43, 221–226. [Google Scholar] [CrossRef]
- Blank, I.; SEn, A.; Grosch, W. Aroma Impact Compounds of Arabica and Robusta Coffee Qualitative and Quantitative Investigations; ASIC: Paris, France, 1991; pp. 117–129.
- Katsuno, T.; Kasuga, H.; Kusano, Y.; Yaguchi, Y.; Tomomura, M.; Cui, J.; Yang, Z.; Baldermann, S.; Nakamura, Y.; Ohnishi, T.; et al. Characterisation of odorant compounds and their biochemical formation in green tea with a low temperature storage process. Food Chem. 2014, 148, 388–395. [Google Scholar] [CrossRef]
- Schieberle, P.; Forschungsanstalt, D.; Garching, D. Formation of Furaneol in Heat-Processed Foods. In Flavor Precursors; American Chemical Society: Washington, DC, USA, 1992; Volume 3, pp. 164–174. [Google Scholar]
- Takei, Y.; Yamanishi, T. Flavor components of roasted almond. Agric. Biol. Chem. 1974, 38, 2329–2336. [Google Scholar]
- Bingham, P.M. Odorants in Breast Milk. Arch. Pediatr. Adolesc. Med. 2012, 166, 1074–1075. [Google Scholar] [CrossRef] [PubMed]
- Cerny, C. Original paper Evaluation of potent odorants in roasted beef by aroma extract dilution analysis. Z. Leb. Forsch. 1991, 322–325. [Google Scholar]
- Karagül-Yüceer, Y.; Drake, M.; Cadwallader, K.R. Aroma-Active Components of Nonfat Dry Milk. J. Agric. Food Chem. 2001, 49, 2948–2953. [Google Scholar] [CrossRef] [PubMed]
- Siefarth, C.; Buettner, A. The Aroma of Goat Milk: Seasonal Effects and Changes through Heat Treatment. J. Agric. Food Chem. 2014, 62, 11805–11817. [Google Scholar] [CrossRef] [PubMed]
- Milo, C.; Reineccius, G.A. Identification and Quantification of Potent Odorants in Regular-Fat and Low-Fat Mild Cheddar Cheese. J. Agric. Food Chem. 1997, 45, 3590–3594. [Google Scholar] [CrossRef]
- Nunomura, N.; Sasaki, M.; Asao, Y.; Yokotsuka, T. Isolation and identification of 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone, as a flavor component in shoyu (soy sauce). Agric. Biol. Chem. 1976, 40, 491–495. [Google Scholar] [CrossRef]
- Schieberle, P. Primary odorants of pale lager beer. Z. Leb. Forsch. 1991, 193, 558–565. [Google Scholar] [CrossRef]
- Xu, Y.; Ji, K. Moutai (Maotai): Production and sensory properties. Alcohol. Beverages Sens. Eval. Consum. Res. 2011, 315–330. [Google Scholar] [CrossRef]
- Vandamme, E.J. Bioflavours and fragrances via fungi and their enzymes. Fungal Divers. 2003, 13, 153. [Google Scholar]
- Larsen, M.; Poll, L. Odour thresholds of some important aroma compounds in strawberriesGeruchsschwellen einiger wichtiger Aromastoffe der Erdbeeren. Z. Leb. Forsch. 1992, 195, 120–123. [Google Scholar] [CrossRef]
- Schwab, W. Natural 4-hydroxy-2,5-dimethyl-3(2h)-furanone (furaneol®). Molecules 2013, 18, 6936–6951. [Google Scholar] [CrossRef]
- Hauck, T.; Bru, F.; Schwab, W.; Icrobiol, A.P.P.L.E.N.M. Zygosaccharomyces rouxii: Identification of an Intermediate. Appl. Environ. Microbiol. 2003, 69, 3911–3918. [Google Scholar] [CrossRef]
- Nashalian, O.; Wang, X.; Yaylayan, V.A. Formation of the reduced form of furaneol® (2,5-dimethyl-4-hydroxy-tetrahydrofuran-3-one) during the Maillard reaction through catalysis of amino acid metal salts. Food Chem. 2016, 210, 43–48. [Google Scholar] [CrossRef]
- Dahlen, T.; Hauck, T.; Wein, M.; Schwab, W. 2,5-Dimethyl-4-Hydroxy-3(2H)-Furanone as a Secondary Metabolite from D-Fructose-l,6-Diphosphate Metabolism by Zygosaccharomyces rouxii. J. Biosci. Bioeng. 2001, 91, 352–358. [Google Scholar] [CrossRef]
- Pérez, A.G.; Olías, R.; Olías, J.M.; Sanz, C. Biosynthesis of 4-Hydroxy-2,5-dimethyl-3(2H)-furanone and Derivatives in in Vitro Grown Strawberries. J. Agric. Food Chem. 1999, 47, 655–658. [Google Scholar] [CrossRef]
- Caballero, J.L.; Moyano, E.; Raab, T.; Lo, J.A. FaQR, Required for the Biosynthesis of the Strawberry Flavor an Enone Oxidoreductase. Plant Cell 2006, 3, 1–15. [Google Scholar]
- Uehara, K.; Watanabe, J.; Mogi, Y.; Tsukioka, Y. Identification and characterization of an enzyme involved in the biosynthesis of the 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone in yeast. J. Biosci. Bioeng. 2017, 123, 333–341. [Google Scholar] [CrossRef]
- Guo, D.L.; Zhang, J.Y.; Liu, C.H. Genetic diversity in some grape varieties revealed by SCoT analyses. Mol. Biol. Rep. 2012, 39, 5307–5313. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Shi, Y.; Duan, C. Evolution of the aromatic profile of traditional Msalais wine during industrial production. Int. J. Food Prop. 2019, 22, 911–924. [Google Scholar] [CrossRef]
- Lixia, Z.; Mingfu, G.; Dongqi, G.; Christensen, M.; Xujie, H.; Hongmei, L.; Yingge, F.; Shu, F. Preliminary analysis of yeast communities associated with the spontaneous fermentation of musalais, a traditional alcoholic beverage of Southern Xinjiang, China. S. Afr. J. Enol. Vitic. 2012, 33, 95–104. [Google Scholar] [CrossRef]
- Zhu, L.; Zhen, W.; Wang, L.; Feng, S. Quantitative Descriptive Analysis of Sensory Characteristics of Musalais from A’wati, Xinjiang. Food Sci. 2013, 34, 38–44. [Google Scholar]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Guth, W. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Hauck, T.; Landmann, C.; Raab, T.; Bru, F. Chemical formation of 4-hydroxy-2,5-dimethyl-3 [2H] –furanone from d-fructose 1,6-diphosphate. Carbohydr. Res. 2002, 337, 1185–1191. [Google Scholar] [CrossRef]
- Schieberle, P. The carbon module labeling (CAMOLA) technique: A useful tool for identifying transient intermediates in the formation of Maillard-type target molecules. Ann. N. Y. Acad. Sci. 2005, 1043, 236–248. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.T. Formation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone through methylglyoxal: A maillard reaction intermediate. J. Agric. Food Chem. 2008, 56, 7405–7409. [Google Scholar] [CrossRef]
- Wong, C.; Mazenod, F.P.; Whitesides, G.M. Chemical and Enzymatic Syntheses of 6-Deoxyhexoses. Conversion to 2,5-Dimethyl-4-hydroxy-2,3-dihydrofuran-3-one (Furaneol) and Analogues. J. Org. Chem. 1983, 48, 3493–3497. [Google Scholar] [CrossRef]
- Zabetakis, I.; Gramshaw, J.W.; Obinson, D.S. 2,5-Dimethyl-4-hydroxy-2H-furan-3-one and its derivatives: Analysis, synthesis and biosynthesis-A review. Food Chem. 1999, 65, 139–151. [Google Scholar] [CrossRef]
- Hattotuwagama, C.K.; Drew, M.G.B.; Nursten, H.E. Quantum mechanics studies of the tautomers of dihydrodiacetylformoin, an important Maillard intermediate and the synthesis of furaneol. J. Mol. Struct. Theochem. 2008, 850, 38–47. [Google Scholar] [CrossRef]
- Rapp, A.; Knipser, W.; Engel, L. A typical aroma compounds in grapes and wines from interspecies hybrid vines. I. Strawb. Note. Vitis 1980, 19, 13–23. [Google Scholar]
- Shure, K.B.; Acree, T.E. Changes in the Odor-Active Compounds in Vitis labruscana Cv. Concord during Growth and Development. J. Agric. Food Chem. 1994, 42, 350–353. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sasaki, K.; Tanzawa, F.; Matsuyama, S.; Suzuki, S.; Takata, R.; Saito, H. Impact of harvest timing on 4-hydroxy-2,5-dimethyl-3(2H)-furanone concentration in “Muscat Bailey A” grape berries. Vitis J. Grapevine Res. 2013, 52, 9–11. [Google Scholar]
- Schreier, P. Volatile Constituents from Concord, Niagara (Vitis labrusca, L.) and Elvira (V. labrusca, L. × V. riparia, M.) Grapes. Can. Inst. Food Sci. Technol. J. 1981, 14, 112–118. [Google Scholar] [CrossRef]
- Roscher, R.; Koch, H.; Herderich, M.; Schreier, P.; Schwab, W. Identification of 2,5-Dimethyl-4-hydroxy-3[2H]-furanone fl-d-Glucuronide as the Major lVletabolite of a Strawberry Flavour Constituent in Humans. Food Chem. Toxicol. 1997, 35, 777–782. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Ma, Y.; Li, J. The glycosidic aroma precursors in wine: Occurrence, characterization and potential biological applications. Phytochem. Rev. 2017, 16, 565–571. [Google Scholar] [CrossRef]
- Zabetakis, I.; Holden, M.A. The effect of 6-deoxy-D-fructose on flavour bioformation from strawberry (Fragaria x ananassa, cv. Elsanta) callus cultures. Plant Cell. Tissue Organ Cult. 1996, 45, 25–29. [Google Scholar] [CrossRef]
- Blank, I.; Devaud, S.; Fay, L.B. New Aspects of The Formation Of 3(2h)-Furanones through The Maillard Reaction. Flavour Sci. Recent Dev. 1996, 188–193. [Google Scholar]
- Varela, C.; Pizarro, F.; Agosin, E. Biomass Content Governs Fermentation Rate in Nitrogen-Deficient Wine Musts. Appl. Environ. Microbiol. 2004, 70, 3392–3400. [Google Scholar] [CrossRef]
Sample Availability: Not available.. |

| Msalais Sample | °Brix | pH | Alcohol (%) | Furaneol | ||
|---|---|---|---|---|---|---|
| Content (mg/L) | OAV1 | OAV2 | ||||
| ms1 | 9.7 ± 0.0c | 3.96 ± 0.01i | 12.3 ± 0.1gh | 81.84 ± 0.00d | 818 | 16,368 |
| ms2 | 12.7 ± 0.0f | 3.66 ± 0.03e | 9.0 ± 0.1bc | 56.51 ± 0.07b | 565 | 11,301 |
| ms3 | 11.8 ± 0.1e | 3.61 ± 0.01d | 8.8 ± 0.6ab | 68.20 ± 0.41fc | 682 | 13,639 |
| ms4 | 13.0 ± 0.1g | 3.10 ± 0.01a | 9.5 ± 0.5cd | 63.50 ± 0.28c | 635 | 12,700 |
| ms5 | 10.8 ± 0.0d | 3.15 ± 0.03b | 8.8 ± 0.0ab | 27.59 ± 0.49a | 276 | 5518 |
| ms6 | 11.0 ± 0.0de | 4.00 ± 0.01j | 10.6 ± 0.2e | 102.73 ± 0.63f | 1027 | 20,545 |
| ms7 | 10.4 ± 0.1cd | 3.56 ± 0.01c | 12.1 ± 0.0g | 53.42 ± 0.21b | 534 | 10,684 |
| ms8 | 7.8 ± 0.0ab | 3.85 ± 0.01g | 9.7 ± 0.1d | 78.14 ± 0.54d | 781 | 15,629 |
| ms9 | 14.3 ± 0.0h | 3.73 ± 0.01f | 8.1 ± 0.1a | 83.17 ± 0.71d | 832 | 16,634 |
| ms10 | 7.0 ± 0.1a | 3.93 ± 0.01h | 9.4 ± 0.1c | 117.6 ± 0.24g | 1176 | 23,520 |
| ms11 | 9.0 ± 0.0bc | 4.09 ± 0.01k | 11.2 ± 0.2f | 91.02 ± 0.85e | 910 | 18,204 |
| ms12 | 10.3 ± 0.0cd | 3.93 ± 0.01h | 10.4 ± 0.1de | 83.66 ± 0.36d | 837 | 16,732 |
| ms13 | 8.3 ± 0.0b | 3.93 ± 0.01g | 10.6 ± 0.1e | 98.47 ± 0.70f | 985 | 19,693 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.-X.; Zhang, M.-M.; Liu, Z.; Shi, Y.; Duan, C.-Q. Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation during Msalais Winemaking. Molecules 2019, 24, 3104. https://doi.org/10.3390/molecules24173104
Zhu L-X, Zhang M-M, Liu Z, Shi Y, Duan C-Q. Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation during Msalais Winemaking. Molecules. 2019; 24(17):3104. https://doi.org/10.3390/molecules24173104
Chicago/Turabian StyleZhu, Li-Xia, Meng-Meng Zhang, Zheng Liu, Yin Shi, and Chang-Qing Duan. 2019. "Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation during Msalais Winemaking" Molecules 24, no. 17: 3104. https://doi.org/10.3390/molecules24173104
APA StyleZhu, L.-X., Zhang, M.-M., Liu, Z., Shi, Y., & Duan, C.-Q. (2019). Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation during Msalais Winemaking. Molecules, 24(17), 3104. https://doi.org/10.3390/molecules24173104






