Aggregation-Morphology-Dependent Electrochemical Performance of Co3O4 Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
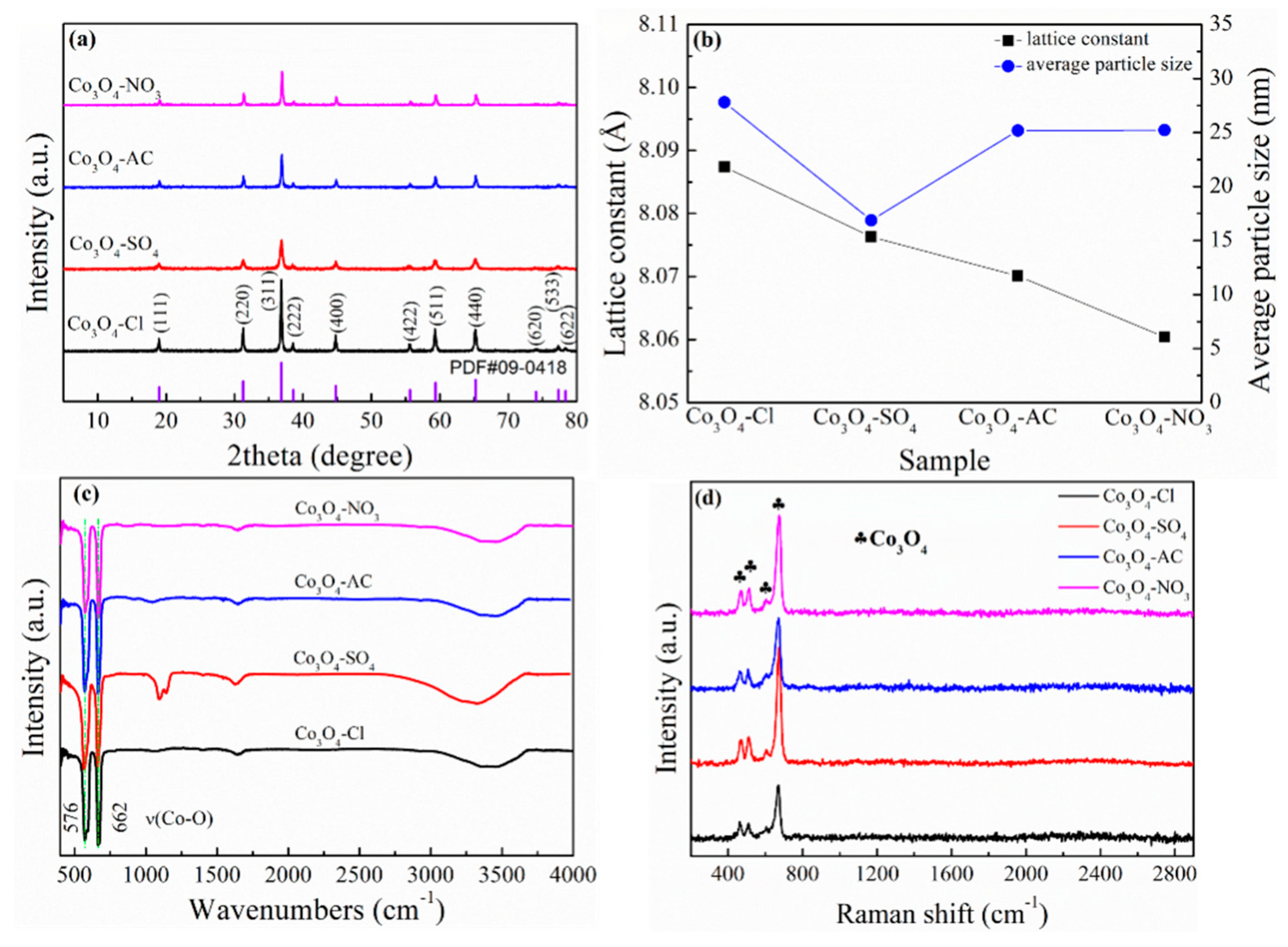
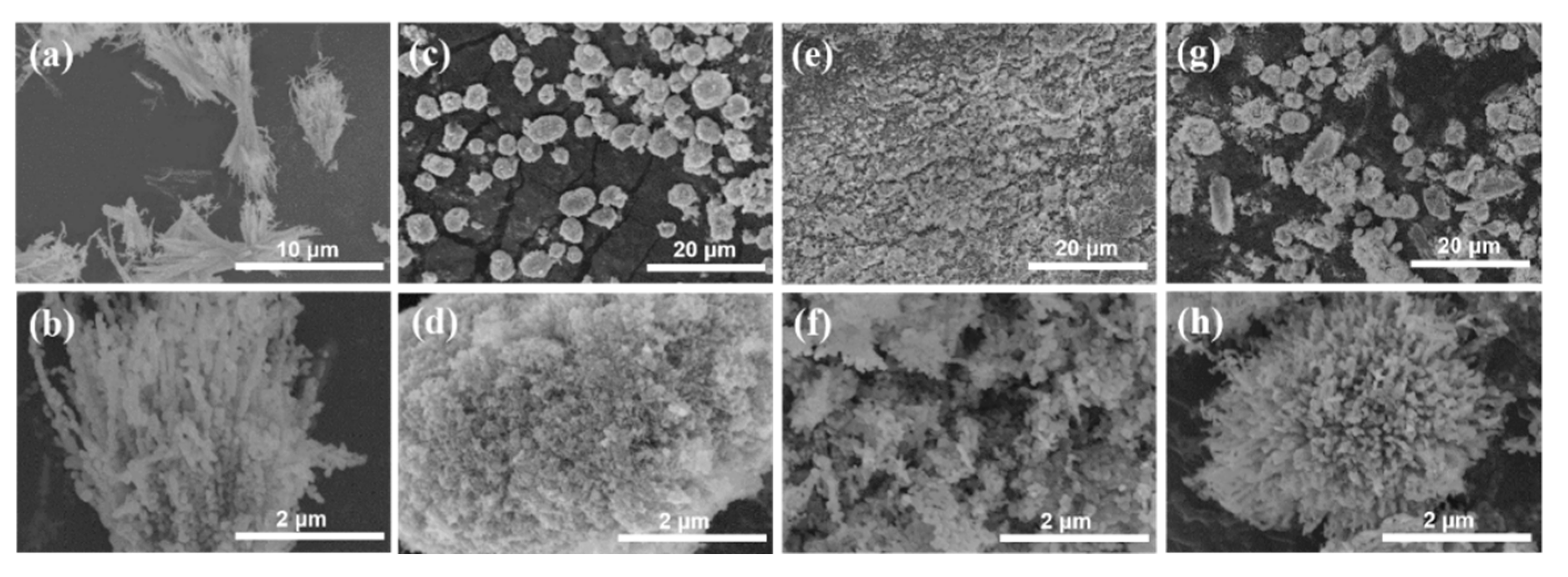
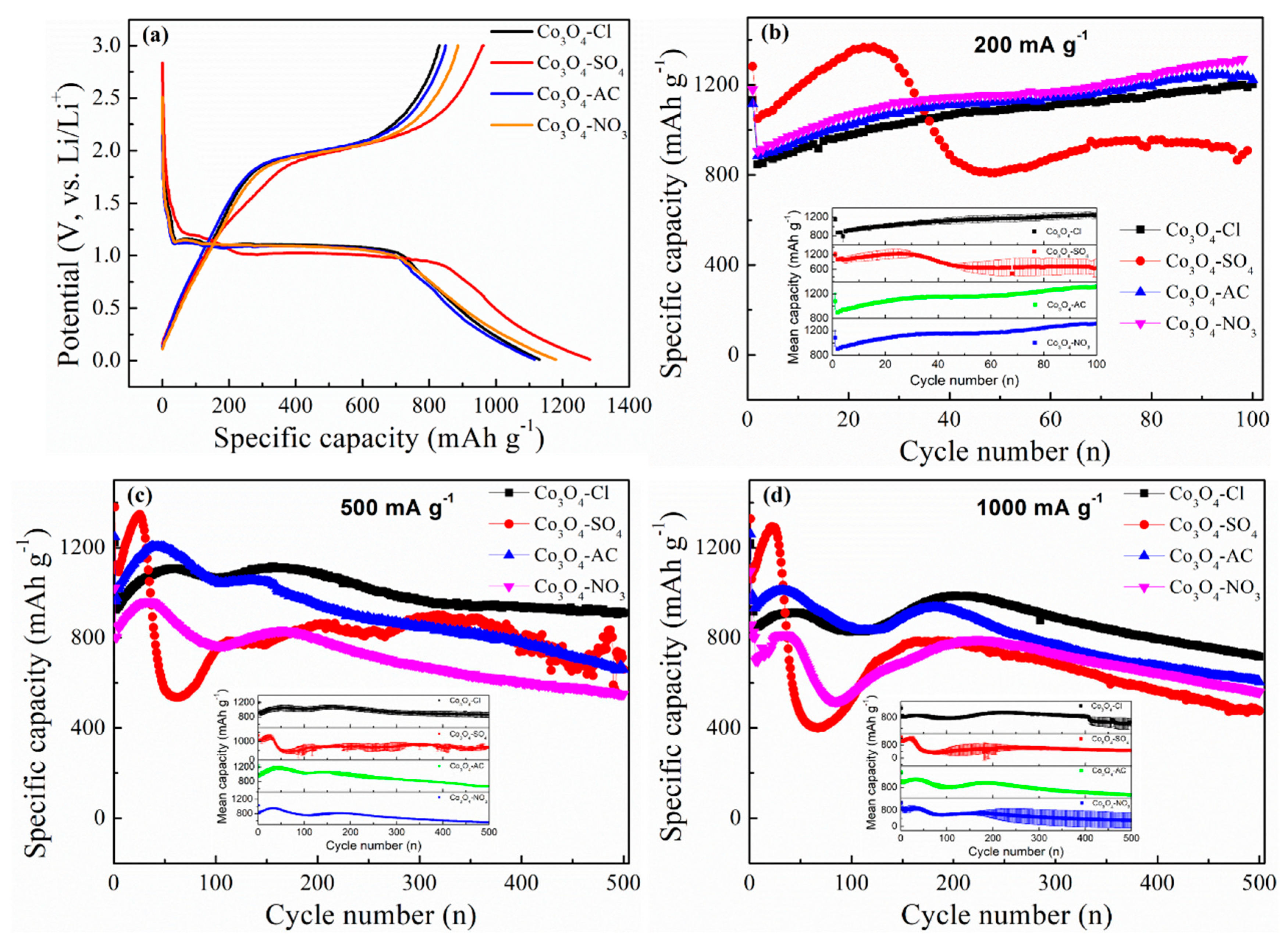
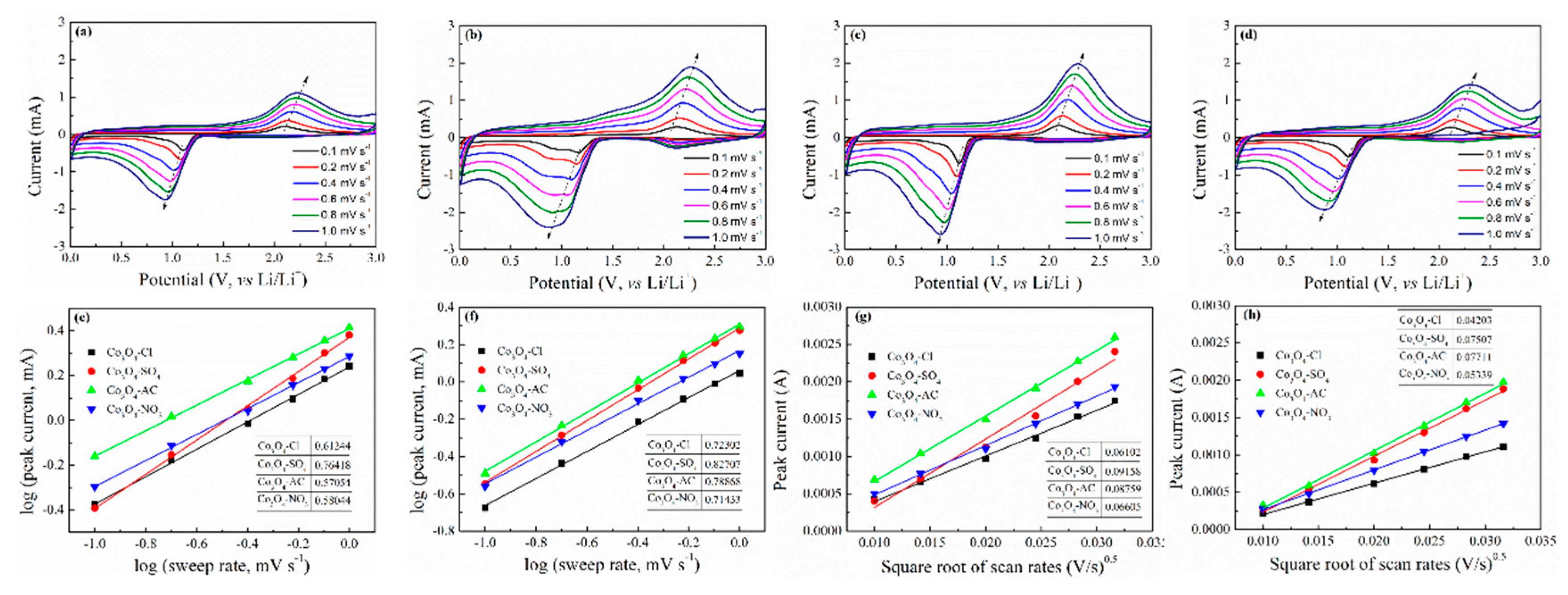
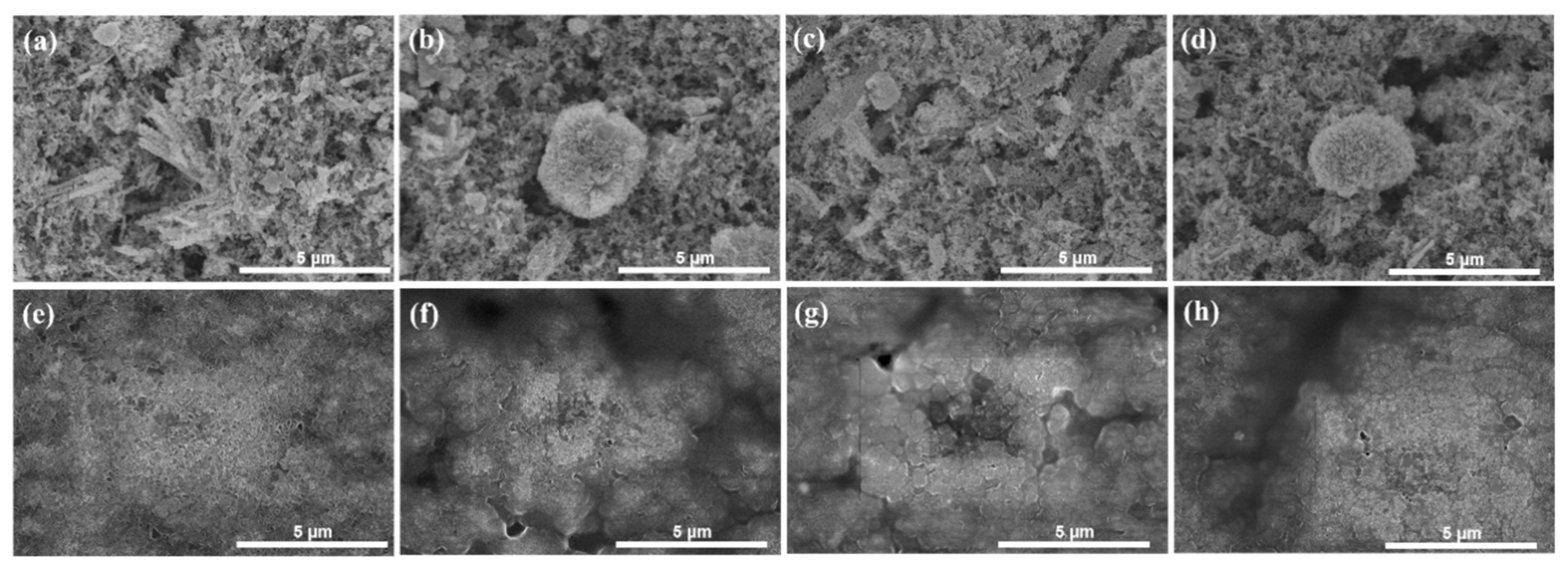
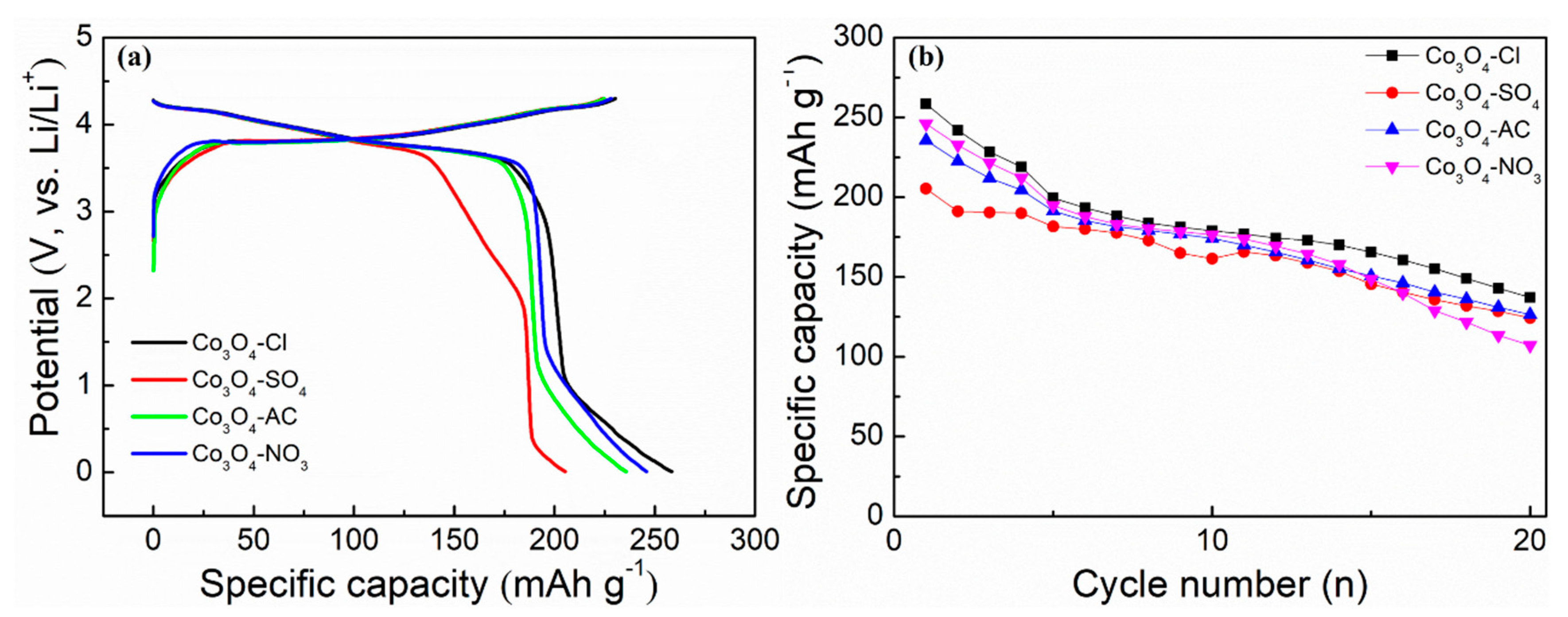
2. Experimental Results and Discussion
3. Experimental Materials and Methods
3.1. Experimental Materials
3.2. Experimental Methods
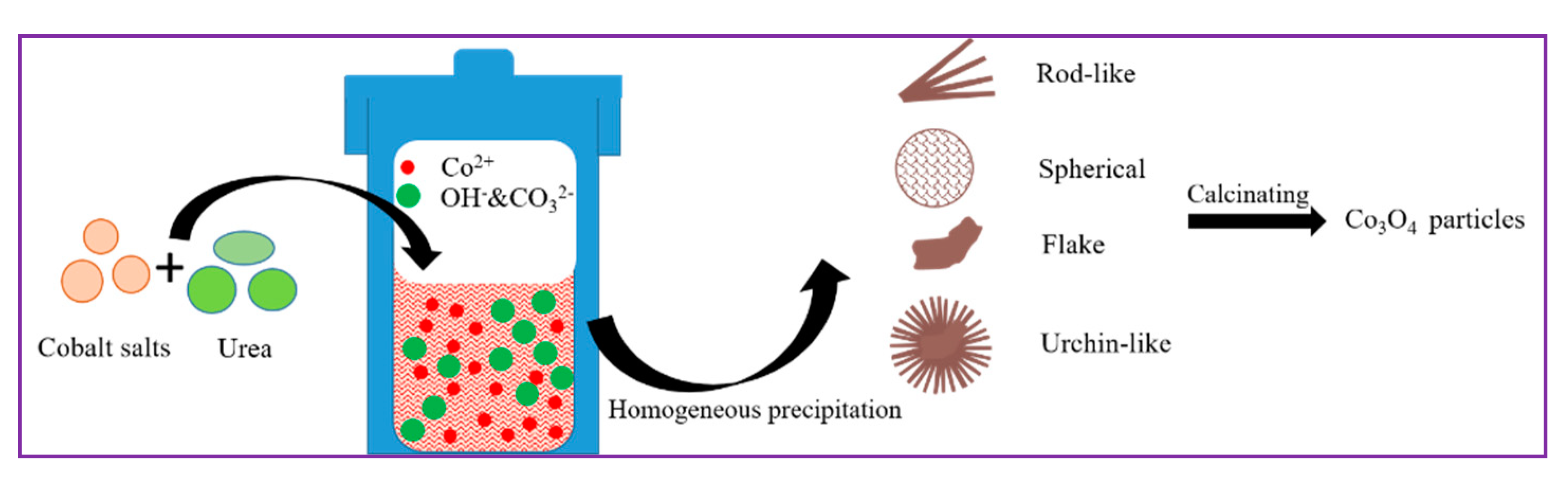
3.2.1. Synthesis of the Precursors
3.2.2. Synthesis of Cobalt Oxides
3.2.3. Material Characterization
3.2.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, H.; Zou, R.; Mahmood, A.; Liang, Z.; Wang, Q.; Zhang, H.; Gao, S.; Qu, C.; Guo, W.; Guo, S.; et al. A Universal Strategy for Hollow Metal Oxide Nanoparticles Encapsulated into B/N Co-Doped Graphitic Nanotubes as High-Performance Lithium-Ion Battery Anodes. Adv. Mater. 2018, 30, 1705441. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Yang, H.X. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar] [CrossRef]
- Trang, V.T.; Rai, A.K.; Gim, J.; Jaekook, K. High performance of Co-doped NiO nanoparticle anode material for rechargeable lithium ion batteries. J. Pow. Sour. 2015, 292, 23–30. [Google Scholar]
- Zheng, Z.; Zao, Y.; Zhang, Q.; Cheng, Y.; Chen, H.; Zhang, K.; Wang, M.; Peng, D. Robust erythrocyte-like Fe2O3@ carbon with yolk-shell structures as high-performance anode for lithium ion batteries. Chem. Eng. J. 2018, 347, 563–573. [Google Scholar] [CrossRef]
- Yue, H.; Shi, Z.; Wang, Q.; Cao, Z.; Dong, H.; Qiao, Y.; Yin, Y.; Yang, S. MOF-derived cobalt-doped ZnO@C composites as a high-performance anode material for lithium-ion batteries. ACS Appl. Mater. Inter. 2014, 6, 17067–17074. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, G.; Gu, H.; Yang, Z.; Cheng, H. A new lithium-ion battery: CuO nanorod array anode versus spinel LiNi0.5Mn1.5O4 cathode. J. Pow. Sour. 2015, 273, 561–565. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of conformal nanoscale MnO on graphene as a high-capacity and long-life anode material for lithium ion batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, X.; Li, B.; Zhao, M.; Pang, H. Co3O4 and its composites for high-performance Li-ion batteries. Chem. Eng. J. 2018, 343, 427–446. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, J.; Zhang, Y.; Huang, Y. Microwave-assisted rapid synthesis of mesoporous nanostructured ZnCo2O4 anode materials for high-performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 24303–24308. [Google Scholar] [CrossRef]
- Gao, G.; Wu, H.; Lou, X. Citrate-assisted growth of NiCo2O4 nanosheets on reduced graphene oxide for highly reversible lithium storage. Adv. Energy Mater. 2014, 4, 1400422. [Google Scholar] [CrossRef]
- Andre, D.; Hain, H.; Lamp, P.; Maglia, F.; Stiaszny, B. Future high-energy density anode materials from an automotive application perspective. J. Mater. Chem. A 2017, 5, 17174–17198. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, R.M. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Energy Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef] [PubMed]
- Meister, P.; Jia, H.; Li, J.; Kloepsch, R.; Winter, M.; Placke, T. Best practice: performance and cost evaluation of lithium ion battery active materials with special emphasis on energy efficiency. Chem. Mater. 2016, 28, 7203–7217. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Li, Q.; Sun, G.; Wang, E.; Li, S.; Gu, J.; Ju, M. Porous cobalt oxide (Co3O4) nanorods: Facile syntheses, optical property and application in lithium-ion batteries. J. Solid State Chem. 2009, 182, 3177–3182. [Google Scholar] [CrossRef]
- Chen, M.; Xia, X.; Yin, J.; Chen, Q. Construction of Co3O4 nanotubes as high-performance anode material for lithium ion batteries. Electrochim. Acta 2015, 160, 15–21. [Google Scholar] [CrossRef]
- Song, H.; Shen, L.; Wang, C. Template-free method towards quadrate Co3O4 nanoboxes from cobalt coordination polymer nano-solids for high performance lithium ion battery anodes. J. Mater. Chem. A 2014, 2, 20597–20604. [Google Scholar] [CrossRef]
- Zhan, F.; Geng, B.; Guo, Y. Porous Co3O4 nanosheets with extraordinarily high discharge capacity for lithium batteries. Chem. A Eur. J. 2009, 15, 6169–6174. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xin, X.; Zhang, Y.; Wang, J.; Liu, Z.; Xu, X. Co3O4 nanowires as high capacity anode materials for lithium ion batteries. J. Alloys Compd. 2012, 521, 95–100. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Wang, Z.; Guo, H.; Hu, Q.; Peng, W. Synthesis of nanoparticles-assembled Co3O4 microspheres as anodes for Li-ion batteries by spray pyrolysis of CoCl2 solution. Electrochim. Acta 2016, 209, 456–463. [Google Scholar] [CrossRef]
- Jang, K.; Hwang, D.K.; Auxili, F.M.; Jang, J.; Song, H.; Oh, B.Y.; Kim, Y.; Nam, J.; Park, J.W.; Jeong, S.; et al. Sub-10-nm Co3O4 nanoparticles/graphene composites as high-performance anodes for lithium storage. Chem. Eng. J. 2017, 309, 15–21. [Google Scholar] [CrossRef]
- Huang, G.; Xu, S.; Lu, S.; Li, L.; Sun, H. Porous polyhedral and fusiform Co3O4 anode materials for high-performance lithium-ion batteries. Electrochim. Acta 2014, 135, 420–427. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, B.; Guo, J.; Zhang, W.; Guo, X. Selective crystal facets exposing of dumbbell-like Co3O4 towards high performances anode materials in lithium-ion batteries. Mater. Res. Bull. 2016, 83, 414–422. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Deng, X.; He, C.; Liu, E.; He, F.; Shi, C.; Zhao, N. Ultrathin-Nanosheet-induced synthesis of 3D transition metal oxides networks for lithium ion battery anodes. Adv. Funct. Mater. 2017, 27, 1605017. [Google Scholar] [CrossRef]
- Xia, X.; Tu, J.; Xiang, J.; Huang, X.; Wang, X.; Zhao, X. Hierarchical porous cobalt oxide array films prepared by electrodeposition through polystyrene sphere template and their applications for lithium ion batteries. J. Pow. Sour. 2010, 195, 2014–2022. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, L.C.; Jiang, Z.B.; Ma, L. Sol-gel synthesis of mesoporous Co3O4 octahedra toward high-performance anodes for lithium-ion batteries. Electrochim. Acta 2014, 129, 410–415. [Google Scholar] [CrossRef]
- Wang, J.; Yang, N.; Tang, H.; Dong, Z.; Jin, Q.; Yang, M.; Kisailus, D.; Zhao, H.; Tang, Z.; Wang, D. Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries. Angew. Chem. Int. Edit. 2013, 52, 6417–6420. [Google Scholar] [CrossRef]
- Tang, X.; Feng, Q.; Huang, J.; Liu, K.; Luo, X.; Peng, Q. Carbon-coated cobalt oxide porous spheres with improved kinetics and good structural stability for long-life lithium-ion batteries. J. Colloid Inter. Sci. 2018, 510, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Kong, X.; Huang, X.; Shi, B. Increasing rigidness of carbon coating for improvement of electrochemical performances of Co3O4 in Li-ion batteries. Carbon 2016, 104, 1–9. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, H.; Fang, J.; Wang, S.; Ding, L.X.; Li, Z.; Ashman, P.J.; Wang, H. Coaxial Co3O4@polypyrrole core-shell nanowire arrays for high performance lithium ion batteries. Electrochim. Acta 2009, 10, 192–200. [Google Scholar]
- Wang, J.; Zhou, H.; Zhu, M.; Yuan, A.; Shen, X. Metal-organic framework-derived Co3O4 covered by MoS2 nanosheets for high-performance lithium-ion batteries. J. Alloys Compd. 2018, 744, 220–227. [Google Scholar] [CrossRef]
- Yan, C.; Chen, G.; Zhou, X.; Sun, J.; Lv, C. Template-based engineering of carbon-doped Co3O4 hollow nanofibers as anode materials for Lithium-ion batteries. Adv. Funct. Mater. 2016, 26, 1428–1436. [Google Scholar] [CrossRef]
- Anh, L.T.; Rai, A.K.; Thi, T.V.; Gim, J.; Kim, S.; Mathew, V.; Kim, J. Enhanced electrochemical performance of novel K-doped Co3O4 as the anode material for secondary lithium-ion batteries. J. Mater. Chem. A 2014, 2, 6966–6975. [Google Scholar] [CrossRef]
- Han, Y.; Li, J.; Zhang, T.; Qi, P.; Li, S.; Gao, X.; Zhou, J.; Feng, X.; Wang, B. Zinc/nickel-doped hollow core–shell Co3O4 derived from a metal–organic framework with high capacity, stability, and rate performance in lithium/sodium-ion batteries. Chem. A Eur. J. 2018, 24, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Jiang, C.; Xu, W.; Cui, X.; Reyes, C.; Martíb, A.; Wang, Y. Facile self-assembly route to Co3O4 nanoparticles confined into single-walled carbon nanotube matrix for highly reversible lithium storage. Electrochim. Acta 2017, 235, 613–622. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Chang, J.; Hu, N.; Xu, C. Self-supporting Co3O4/graphene hybrid films as binder-free anode materials for lithium ion batteries. Sci. Rep. 2018, 8, 3182. [Google Scholar] [CrossRef]
- Shi, L.; Fan, C.; Fu, X.; Yu, S.; Qian, G.; Wang, Z. Carbonate-assisted hydrothermal synthesis of porous hierarchical Co3O4/CuO composites as high capacity anodes for lithium-ion batteries. Electrochim. Acta 2016, 197, 23–31. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Yin, L.; Qi, Y. Prussion blue-supported annealing chemical reaction route synthesized double-shelled Fe2O3/Co3O4 hollow microcubes as anode materials for lithium-ion battery. ACS Appl. Mater. Inter. 2014, 6, 8098–8107. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wei, L. Synthesis of ultrafine Co3O4 nanoparticles encapsulated in nitrogen-doped porous carbon matrix as anodes for stable and long-life lithium ion battery. J. Alloys Compd. 2019, 790, 955–962. [Google Scholar] [CrossRef]
- Wang, B.; Lu, X.Y.; Tsang, C.-W.; Wang, Y.; Au, W.K.; Guo, H.; Tang, Y. Charge-driven self-assembly synthesis of straw-sheaf-like Co3O4 with superior cyclability and rate capability for lithium-ion batteries. Chem. Eng. J. 2018, 338, 278–286. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Horvat, J.; Wang, B.; Liu, H.; Wexler, D.; Yao, J. Hydrothermal synthesis and optical, magnetic, and supercapacitance properties of nanoporous cobalt oxide nanorods. J. Phys. Chem. C 2009, 113, 4357–4361. [Google Scholar] [CrossRef]
- Dong, X.; Xu, H.; Wang, X.; Huang, Y.; Chan-Park, M.B.; Zhang, H.; Wang, L.; Huang, W.; Chen, P. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Zhou, M.; Li, G.; Chen, Z.; Xu, W.; Chen, X.; Hou, Z. Graphene-embedded Co3O4 rose-spheres for enhanced performance in lithium ion batteries. ACS Appl. Mater. Inter. 2017, 9, 9662–9668. [Google Scholar] [CrossRef]
- Deng, X.; Zhu, S.; He, F.; Liu, E.; He, C.; Shi, C.; Li, Q.; Li, J.; Ma, L.; Zhao, N. Three-dimensionally hierarchical Co3O4/Carbon composites with high pseudocapacitance contribution for enhancing lithium storage. Electrochim. Acta 2018, 283, 1269–1276. [Google Scholar] [CrossRef]
- Hao, F.; Zhang, Z.; Yin, L. Co3O4/carbon aerogel hybrids as anode materials for lithium-ion batteries with enhanced electrochemical properties. ACS Appl. Mater. Inter. 2013, 5, 8337–8344. [Google Scholar] [CrossRef]
- Gan, Q.; Zhao, K.; He, Z.; Liu, S.; Li, A. Zeolitic imidazolate framework-8-derived N-doped porous carbon coated olive-shaped FeOx nanoparticles for lithium storage. J. Pow. Sour. 2018, 384, 187–195. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, L.; Lin, H.; Ma, L.; Zhao, P.; Hu, Y.; Chen, T.; Chen, R.; Wang, Y.; Tie, Z.; et al. Walnut-like multicore–shell MnO encapsulated nitrogenrich carbon nanocapsules as anode material for longcycling and soft-packed lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1800003. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Qiu, B.; Zhou, S.; Zhang, M.; Huang, H.; Wang, B.; Zhao, J.; Sun, X.; Qiu, J. Ultrahigh rate and long-life sodium-ion batteries enabled by engineered surface and near-surface reactions. Adv. Mater. 2018, 30, 1702486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Zhang, M.; Qian, Y. Synthesis of Co3O4 nanorod bunches from a single precursor Co(CO3)0.35Cl0.20(OH)1. Solid State Sci. 2005, 7, 13–15. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


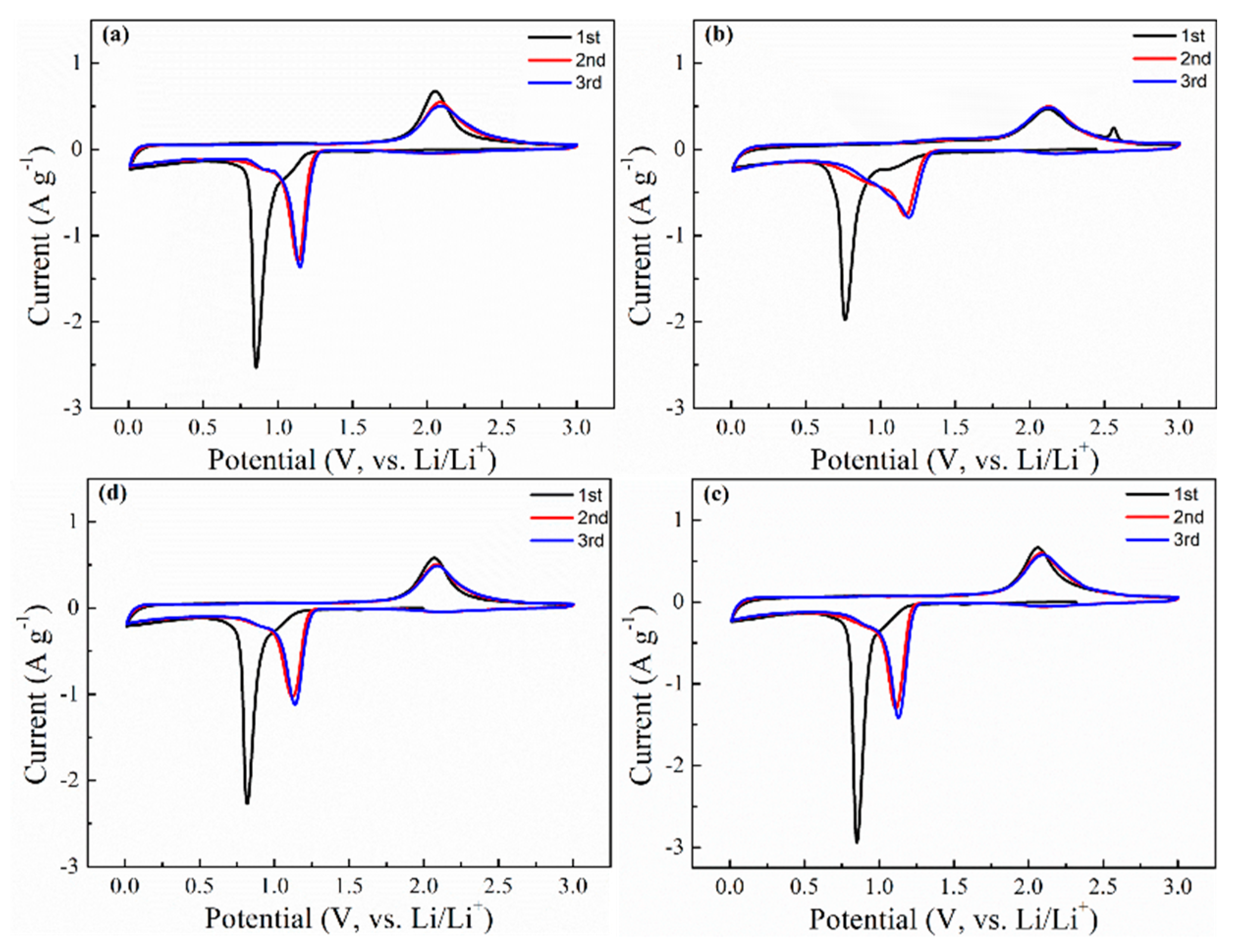


| Sample | 1st Cycle | 2nd Cycle | 3rd Cycle | |||
|---|---|---|---|---|---|---|
| ER (V) | EO (V) | ER (V) | EO (V) | ER (V) | EO (V) | |
| Co3O4-Cl | 0.854 | 2.055 | 1.135 | 2.085 | 1.146 | 2.094 |
| Co3O4-SO4 | 0.765 | 2.118 | 1.166 | 2.127 | 1.190 | 2.123 |
| Co3O4-AC | 0.850 | 2.061 | 1.111 | 2.085 | 1.129 | 2.097 |
| Co3O4-NO3 | 0.817 | 2.070 | 1.120 | 2.084 | 1.138 | 2.092 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Wang, L.; Sun, D.; Meng, S.; Xu, D.; He, Z.; Dong, X.; Li, Y.; Jin, Y. Aggregation-Morphology-Dependent Electrochemical Performance of Co3O4 Anode Materials for Lithium-Ion Batteries. Molecules 2019, 24, 3149. https://doi.org/10.3390/molecules24173149
Kong L, Wang L, Sun D, Meng S, Xu D, He Z, Dong X, Li Y, Jin Y. Aggregation-Morphology-Dependent Electrochemical Performance of Co3O4 Anode Materials for Lithium-Ion Batteries. Molecules. 2019; 24(17):3149. https://doi.org/10.3390/molecules24173149
Chicago/Turabian StyleKong, Linglong, Lu Wang, Deye Sun, Su Meng, Dandan Xu, Zaixin He, Xiaoying Dong, Yongfeng Li, and Yongcheng Jin. 2019. "Aggregation-Morphology-Dependent Electrochemical Performance of Co3O4 Anode Materials for Lithium-Ion Batteries" Molecules 24, no. 17: 3149. https://doi.org/10.3390/molecules24173149






