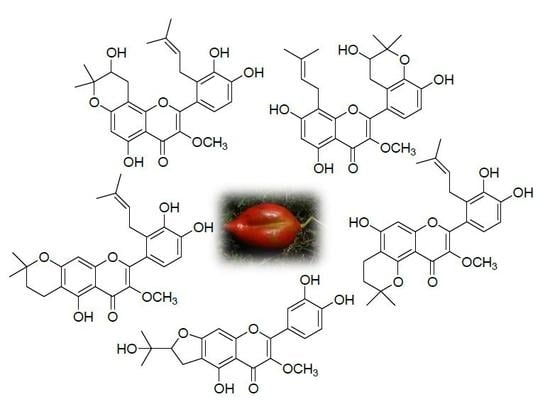
Sixteen New Prenylated Flavonoids from the Fruit of Sinopodophyllum hexandrum
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic and Physical Data
3.5. Cytotoxicity Asssay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, C.Q.; Cao, W.; Nagatsu, A.; Ogihara, Y. Three new glycosides from Sinopodophyllum emodi (Wall.) Ying. Chem. Pharm. Bull. 2001, 49, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Xiao, J.J.; Meng, S.C.; Dong, X.M.; Ge, Y.W.; Wang, R.F.; Shang, M.Y.; Cai, S.Q. A new cytotoxic flavonoid from the fruit of Sinopodophyllum hexandrum. Fitoterapia 2010, 81, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Zhu, Y.Y.; Chen, S.Y.; Ogihara, Y. Lignan glucoside from Sinopodophyllum emodi and its cytotoxic activity. Chin. Chem. Lett. 2011, 22, 181–184. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Huang, J.; Nagatsu, A.; Ogihara, Y. Two new podophyllotoxin glycosides from Sinopodophyllum emodi (Wall) Ying. Chem. Pharm. Bull. 2001, 49, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Nagatsu, A.; Hatano, K.; Shirai, N.; Kato, S.; Ogihara, Y. New lignan glycosides from Chinese medicinal plant, Sinopodophyllum emodi. Chem. Pharm. Bull. 2003, 51, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Liu, X.Q.; Zhou, W.; Hua, H.M. Three new cytotoxic aryltetralin lignans from Sinopodophyllum emodi. Bioorg. Med. Chem. Lett. 2011, 21, 3794–3797. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Liu, X.Q.; Zhou, W.; Hua, H.M. Four new cytotoxic tetrahydrofuranoid lignans from Sinopodophyllum emodi. Planta Med. 2012, 78, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Zhou, W.; Chen, H.; Li, Z.L.; Hua, H.M. Isolation and identification of flavonoids from the roots and rhizomes of Sinopodophyllum emodi. J. Shenyang Pharm. Univ. 2012, 29, 185–189. [Google Scholar]
- Sun, Y.J.; Pei, L.X.; Wang, K.B.; Sun, Y.S.; Wang, J.M.; Zhang, Y.L.; Gao, M.L.; Ji, B.Y. Preparative isolation of two prenylated biflavonoids from the roots and rhizomes of Sinopodophyllum emodi by sephadex LH-20 column and high-speed counter-current chromatography. Molecules 2016, 21, 10. [Google Scholar] [CrossRef]
- Sun, Y.J.; Sun, Y.S.; Chen, H.; Hao, Z.Y.; Wang, J.M.; Guan, Y.B.; Zhang, Y.L.; Feng, W.S.; Zheng, X.K. Isolation of two new prenylated flavonoids from Sinopodophyllum emodi fruit by silica gel column and high-speed counter-current chromatography. J. Chromatogra. B 2014, 969, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Hao, Z.Y.; Si, J.G.; Wang, Y.; Zhang, Y.L.; Wang, J.M.; Gao, M.L.; Chen, H. Prenylated flavonoids from the fruits of Sinopodophyllum emodi and their cytotoxic activities. RSC Adv. 2015, 5, 82736–82742. [Google Scholar] [CrossRef]
- Sun, Y.J.; Gao, M.L.; Zhang, Y.L.; Wang, J.M.; Wu, Y.; Wang, Y.; Liu, T. Labdane diterpenes from the fruits of Sinopodophyllum emodi. Molecules 2016, 21, 434. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Zhou, W.; Hua, H.M. Study on chemical constituents from the roots and rhizomes of Sinopodophyllum emodi. J. Chin. Med. Mat. 2012, 35, 1607–1609. [Google Scholar]
- Sun, Y.J.; Zhou, W.; Chen, H.; Li, Z.L.; Hua, H.M. Phenols from roots and rhizomes of Sinopodophyllum emodi. Chin. Tradit. Herbal Drugs 2012, 43, 226–229. [Google Scholar]
- Yang, X.Z.; Shao, H.; Zhang, L.Q.; Zhou, C.; Xuan, Q.; Yang, C.Y. Present situation of studies on resources of podophyllotoxin. Chin. Tradit. Herbal Drugs 2001, 32, 1042–1044. [Google Scholar]
- Kirmizibekmez, H.; Uysal, G.B.; Masullo, M.; Demirci, F.; Bagci, Y.; Kan, Y.; Piacente, S. Prenylated polyphenolic compounds from Glycyrrhiza iconica and their antimicrobial and antioxidant activities. Fitoterapia 2015, 103, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.P.; Xiang, J.M. A new cyclodemethylanhydroicaritin from extraction of Epimedium wushanense and evaluation of its biological activities. Chem. Nat. Compd. 2016, 52, 791–793. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, W.Z.; Zhang, C.M.; Xiang, N.G.; Liu, C.B.; Yang, G.Y.; Chen, Y.K.; Miao, M.M.; Ma, Y.H. Antiviral flavones from the leaves of Nicotiana tabacum. Phytochem. Lett. 2015, 12, 75–78. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, Y.G.; Zhang, Y.; Li, T.; Li, H.L.; Yan, S.K.; Jin, H.Z.; Zhang, W.D. New norlignans and flavonoids of Dysosma versipellis. Phytochem. Lett. 2016, 16, 75–81. [Google Scholar] [CrossRef]
- Zhao, L.; Peng, X.J.; Xia, P.F.; Duan, W.D. Chemical constituents of Tamarix chinensis. J. Chin. Med. Mat. 2014, 37, 61–63. [Google Scholar]
- Shen, S.M.; Shen, L.G.; Lei, Q.F.; Si, J.Y.; Liu, C.M.; Lu, H. Chemical constituents contained in aerial parts of Emilia sonchifolia. Chin. J. Chin. Mater. Med. 2012, 37, 3249–3251. [Google Scholar]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Zarev, Y.; Foubert, K.; Lucia de Almeida, V.; Anthonissen, R.; Elgorashi, E.; Apers, S.; Ionkova, I.; Verschaeve, L.; Pieters, L. Antigenotoxic prenylated flavonoids from stem bark of Erythrina latissima. Phytochemistry 2017, 141, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Tuenter, E.; Zarev, Y.; Matheeussen, A.; Elgorashi, E.; Pieters, L.; Foubert, K. Antiplasmodial prenylated flavonoids from stem bark of Erythrina latissima. Phytochem. Lett. 2019, 30, 169–172. [Google Scholar] [CrossRef]
- Indran, I.R.; Zhang, S.J.; Zhang, Z.W.; Sun, F.; Gong, Y.H.; Wang, X.C.; Li, J.; Erdelmeier, C.A.J.; Koch, E.; Yong, E.L. Selective estrogen receptor modulator effects of Epimedium extracts on breast cancer and uterine growth in nude mice. Planta Med. 2014, 80, 22–28. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 6 | 6.27 s | 6.31 s | 6.29 s | |||||
| 8 | 6.35 s | 6.28 s | 6.34 s | 6.40 s | 6.53 s | |||
| 2′ | 7.46 d (2.2) | 7.54 d (2.2) | ||||||
| 5′ | 6.70 d (8.2) | 6.76 d (8.3) | 6.68 d (8.2) | 6.73 d (8.4) | 6.86 d (8.5) | 6.90 d (8.5) | 6.90 d (8.4) | 6.79 d (8.4) |
| 6′ | 6.74 d (8.2) | 6.74 d (8.3) | 6.72 d (8.2) | 6.76 d (8.4) | 7.35 dd (8.5, 2.2) | 7.43 dd (8.5, 2.2) | 7.59 d (8.4) | 7.09 d (8.4) |
| 1″ | 3.21 d (6.8) | 3.21 d (7.1) | 2.54 d (6.8) | 2.62 t (6.7) | 2.54 t (6.7) | 3.06 d (8.4) | 3.30 d (6.8) | 3.30 d (6.8) |
| 2″ | 5.01 t (6.8) | 5.16 t (7.1) | 1.74 t (6.8) | 1.81 t (6.7) | 1.72 t (6.7) | 4.75 t (8.4) | 5.06 t (6.8) | 5.05 t (6.8) |
| 4″ | 1.45 s | 1.61 s | 1.30 s | 1.30 s | 1.30 s | 1.13 s | 1.49 s | 1.58 s |
| 5″ | 1.53 s | 1.71 s | 1.30 s | 1.30 s | 1.30 s | 1.14 s | 1.56 s | 1.60 s |
| 1‴ | 3.25 d (6.8) | 3.24 d (7.3) | 3.21 d (6.9) | 3.25 d (6.9) | 7.05 d (2.1) | 3.44 dd (17.1, 6.8) 2.95 dd (17.1, 2.4) | ||
| 2‴ | 4.94 t (6.8) | 5.03 t (7.3) | 5.04 t (6.9) | 5.00 t (6.9) | 8.09 d (2.1) | 6.03 br.s | ||
| 4‴ | 1.27 s | 1.34 s | 1.34 s | 1.30 s | ||||
| 5‴ | 1.39 s | 1.49 s | 1.50 s | 1.45 s | ||||
| OCH3 | 3.56 s | 3.48 s | 3.56 s | 3.69 s | 3.77 s | 3.65 s | 3.64 s | |
| 5-OH | 12.46 s | 12.90 s | 13.00 s | 12.92 s | 12.70 s | 12.65 s | ||
| 7-OH | 10.64 s | 10.81 s | 10.60 s | 10.67 s | 10.84 s | |||
| No. | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 6 | 6.17 s | 6.30 s | 6.30 s | 6.18 s | 6.12 s | 6.17 s | 6.16 s | 6.30 s |
| 8 | 6.43 s | |||||||
| 2′ | 7.80 d (2.0) | 7.73 d (2.1) | ||||||
| 5′ | 6.77 s | 6.75 d (8.2) | 6.90 d (8.4) | 6.87 d (8.5) | 6.89 d (8.5) | 6.75 d (8.5) | 6.76 d (8.2) | 6.75 d (8.2) |
| 6′ | 6.77 s | 6.87 d (8.2) | 7.55 d (8.4) | 7.78 d (8.5, 2.0) | 7.60 dd (8.5, 2.1) | 6.92 d (8.5) | 6.89 d (8.2) | 6.90 d (8.2) |
| 1″ | 2.79 dd (16.5, 5.2) 2.47 dd (16.5, 7.1) | 3.23 d (6.9) | 2.67 m | 2.83 d (6.5) | 2.84 t (6.6) | 2.83 dd (16.5, 5.1) 2.54 dd (16.5, 6.7) | 4.20 d (2.7) | 3.13 d (8.5) |
| 2″ | 3.67 dd (7.1, 5.2) | 5.05 t (6.9) | 1.47 m | 1.82 d (6.5) | 1.87 t (6.6) | 3.69 dd (6.7, 5.1) | 3.80 m | 4.74 t (8.5) |
| 4″ | 1.20 s | 1.48 s | 0.98 s | 1.32 s | 1.32 s | 1.23 s | 1.32 s | 1.13s |
| 5″ | 1.27 s | 1.54 s | 0.98 s | 1.32 s | 1.32 s | 1.27 s | 1.36 s | 1.14 s |
| 1‴ | 3.23 d (6.9) | 2.75 dd (17.0, 5.4) 2.55 dd (17.0, 8.2) | 7.01 s | 2.68 t (6.6) | 2.63 m | 2.66 m | ||
| 2‴ | 4.99 t (6.9) | 3.60dd (8.2, 5.4) | 1.73 t (6.6) | 1.71 m | 1.73 m | |||
| 4‴ | 1.27 s | 1.17 s | 5.77 s 5.28 s | 1.32 s | 1.31 s | 1.30 s | ||
| 5‴ | 1.44 s | 1.32 s | 2.10 s | 1.32 s | 1.31 s | 1.31 s | ||
| OCH3 | 3.57 s | 3.57 s | 3.65 s | 3.78 s | 3.58 s | 3.59 s | 3.57 s | |
| OCH3 | 3.31 s | |||||||
| 5-OH | 12.45 s | 12.57 s | 12.67 s | 12.65 s | 12.24 s | 12.44 s | 12.65 s | 12.87 s |
| 7-OH | 10.81 s |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 2 | 150.7 s | 159.6 s | 155.1 s | 159.7 s | 151.4 s | 155.7 s | 156.6 s | 156.0 s |
| 3 | 136.2 s | 139.0 s | 140.6 s | 138.5 s | 139.7 s | 137.7 s | 137.7 s | 138.0 s |
| 4 | 176.5 s | 178.5 s | 172.0 s | 178.2 s | 172.1 s | 178.0 s | 178.1 s | 178.1 s |
| 5 | 158.3 s | 158.5 s | 154.6 s | 158.2 s | 154.5 s | 155.3 s | 160.0 s | 159.0 s |
| 6 | 97.7 d | 111.1 s | 105.0 s | 104.4 s | 105.0 s | 108.8 s | 98.2 d | 98.2 d |
| 7 | 160.8 s | 162.3 s | 159.8 s | 159.8 s | 159.8 s | 166.2 s | 161.6 s | 161.6 s |
| 8 | 105.4 s | 93.3 d | 93.2 d | 94.4 d | 93.1 d | 88.5 d | 106.0 s | 105.9 s |
| 9 | 154.2 s | 154.9 s | 156.4 s | 154.4 s | 156.0 s | 156.2 s | 153.9 s | 153.9 s |
| 10 | 103.5 s | 104.7 s | 107.6 s | 104.3 s | 107.2 s | 105.1 s | 104.3 s | 104.4 s |
| 1′ | 123.0 s | 123.2 s | 121.8 s | 121.1 s | 121.4 s | 120.7 s | 113.4 s | 117.8 s |
| 2′ | 128.1 s | 128.2 s | 127.6 s | 127.8 s | 115.0 d | 115.7 d | 128.1 s | 127.0 s |
| 3′ | 143.0 s | 143.7 s | 143.2 s | 143.3 s | 145.1 s | 145.2 s | 143.1 s | 145.4 s |
| 4′ | 146.5 s | 147.4 s | 146.5 s | 147.1 s | 147.7 s | 148.1 s | 145.5 s | 143.3 s |
| 5′ | 112.4 d | 112.9 d | 112.4 d | 112.4 d | 115.6 d | 115.4 d | 110.4 d | 115.2 d |
| 6′ | 121.0 d | 121.5 d | 120.9 d | 121.0 d | 119.8 d | 120.5 d | 125.9 d | 122.3 d |
| 1″ | 20.9 t | 21.4 t | 16.7 t | 15.7 t | 16.7 t | 25.7 t | 21.1 t | 21.1 t |
| 2″ | 121.99 d | 122.6 d | 30.8 t | 30.9 t | 30.8 t | 91.5 d | 122.4 d | 122.4 d |
| 3″ | 130.6 s | 131.1 s | 74.7 s | 76.2 s | 74.8 s | 70.0 s | 131.0 s | 131.3 s |
| 4″ | 17.3 q | 18.1 q | 26.4 q | 26.3 q | 26.4 q | 24.8 q | 17.6 q | 17.7 q |
| 5″ | 25.4 q | 25.9 q | 26.4 q | 26.3 q | 26.4 q | 25.9 q | 25.3 q | 25.4 q |
| 1‴ | 25.7 t | 26.2 t | 26.0 t | 25.7 q | 107.3 d | 38.2 t | ||
| 2‴ | 122.04 d | 123.3 d | 122.9 d | 122.8 d | 146.6 d | 101.0 d | ||
| 3‴ | 129.8 s | 130.6 s | 129.9 s | 130.2 s | ||||
| 4‴ | 17.2 q | 17.8 q | 17.4 q | 17.4 q | ||||
| 5‴ | 25.2 q | 25.7 q | 25.4 q | 25.4 q | ||||
| OCH3 | 60.3 q | 59.4 q | 59.9 q | 59.2 q | 59.6 q | 60.6 q | 60.2 q | |
| No. | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 2 | 158.7 s | 158.2 s | 156.7 s | 156.6 s | 147.7 s | 158.3 s | 158.0 s | 158.0 s |
| 3 | 139.1 s | 138.7 s | 137.7 s | 138.2 s | 136.1 s | 139.2 s | 139.5 s | 138.8 s |
| 4 | 178.2 s | 178.2 s | 178.9 s | 178.3 s | 175.9 s | 178.1 s | 178.1 s | 178.1 s |
| 5 | 159.4 s | 159.0 s | 158.7 s | 161.6 s | 158.0 s | 158.75 s | 160.3 s | 161.8 s |
| 6 | 98.9 d | 98.2 d | 98.2 d | 99.0 d | 98.5 d | 98.8 d | 98.7 d | 93.3 d |
| 7 | 158.7 s | 161.5 s | 161.7 s | 164.7 s | 159.1 s | 158.82 s | 158.8 s | 166.2 s |
| 8 | 99.0 s | 105.9 s | 107.2 s | 94.1 d | 99.7 s | 99.0 s | 100.3 s | 104.4 s |
| 9 | 154.1 s | 154.1 s | 154.1 s | 155.8 s | 153.0 s | 154.0 s | 156.0 s | 151.3 s |
| 10 | 105.4 s | 104.5 s | 104.4 s | 104.6 s | 103.6 s | 105.5 s | 105.8 s | 105.4 s |
| 1′ | 121.2 s | 120.5 s | 113.5 s | 121.7 s | 122.2 s | 120.3 s | 120.2 s | 120.2 s |
| 2′ | 127.7 s | 120.6 s | 129.4 s | 130.2 d | 114.8 d | 121.3 s | 121.0 s | 121.1 s |
| 3′ | 143.3 s | 141.0 s | 142.7 s | 121.5 s | 145.1 s | 142.0 s | 141.9 s | 142.0 s |
| 4′ | 147.1 s | 147.9 s | 144.9 s | 156.8 s | 146.7 s | 148.3 s | 148.2 s | 148.3 s |
| 5′ | 112.5 d | 112.9 d | 111.0 d | 117.5 d | 115.7 d | 112.3 d | 112.8 d | 112.7 d |
| 6′ | 121.0 d | 121.5 d | 125.9 d | 128.1 d | 120.2 d | 121.1 d | 121.4 d | 121.2 d |
| 1″ | 24.9 t | 21.0 t | 17.5 t | 22.2 t | 15.7 t | 24.7 t | 73.9 d | 25.2 t |
| 2″ | 66.8 d | 122.0 d | 47.8 t | 32.5 t | 30.9 t | 66.6 d | 67.3 d | 91.5 d |
| 3″ | 78.9 s | 130.9 s | 68.7 s | 75.8 s | 76.2 s | 78.9 s | 78.8 s | 69.9 s |
| 4″ | 20.8 q | 17.4 q | 28.8 q | 27.0 q | 26.3 q | 20.2 q | 23.1 q | 24.8 q |
| 5″ | 25.1 q | 25.5 q | 28.8 q | 27.0 q | 26.3 q | 25.2 q | 24.5 q | 25.7 q |
| 1‴ | 25.7 t | 29.5 t | 104.2 d | 21.1 t | 19.9 t | 20.1 t | ||
| 2‴ | 122.9 d | 67.8 d | 156.5 s | 31.8 t | 31.8 t | 31.7t | ||
| 3‴ | 130.2 s | 76.8 s | 132.5 s | 73.9 s | 73.5 s | 73.9 s | ||
| 4‴ | 17.2 q | 19.8 q | 114.1 t | 26.0 q | 26.4 q | 26.2 q | ||
| 5‴ | 25.2 q | 25.3 q | 18.9 q | 26.0 q | 26.4 q | 26.6 q | ||
| OCH3 | 59.8 q | 60.2 q | 60.1 q | 60.1 q | 59.2 q | 60.2 q | 60.2 q | 60.2 q |
| OCH3 | 56.9 q |
| Compound | MCF-7 | HepG2 | Compound | MCF-7 | HepG2 |
|---|---|---|---|---|---|
| 1 | 33.8 ± 2.0 | 75.9 ± 5.3 | 15 | 25.5 ± 1.8 | 17.2 ± 1.3 |
| 2–5, 7–12, 17, 18 | >100 | >100 | 16 | 41.8 ± 3.5 | 55.4 ± 4.9 |
| 6 | 6.25 ± 0.49 | 3.83 ± 0.26 | 20 | 48.3 ± 3.2 | 50.6 ± 4.4 |
| 13 | 59.7 ± 4.1 | 45.3 ± 3.5 | 22 | 59.3 ± 5.7 | >100 |
| 14 | 30.4 ± 2.6 | 23.1 ± 1.7 | 5-fluorouracil | 33.4 ± 3.0 | 18.2 ± 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Chen, H.; Wang, J.; Gao, M.; Zhao, C.; Han, R.; Chen, H.; Li, M.; Xue, G.; Feng, W. Sixteen New Prenylated Flavonoids from the Fruit of Sinopodophyllum hexandrum. Molecules 2019, 24, 3196. https://doi.org/10.3390/molecules24173196
Sun Y, Chen H, Wang J, Gao M, Zhao C, Han R, Chen H, Li M, Xue G, Feng W. Sixteen New Prenylated Flavonoids from the Fruit of Sinopodophyllum hexandrum. Molecules. 2019; 24(17):3196. https://doi.org/10.3390/molecules24173196
Chicago/Turabian StyleSun, Yanjun, Haojie Chen, Junmin Wang, Meiling Gao, Chen Zhao, Ruijie Han, Hui Chen, Meng Li, Guimin Xue, and Weisheng Feng. 2019. "Sixteen New Prenylated Flavonoids from the Fruit of Sinopodophyllum hexandrum" Molecules 24, no. 17: 3196. https://doi.org/10.3390/molecules24173196
APA StyleSun, Y., Chen, H., Wang, J., Gao, M., Zhao, C., Han, R., Chen, H., Li, M., Xue, G., & Feng, W. (2019). Sixteen New Prenylated Flavonoids from the Fruit of Sinopodophyllum hexandrum. Molecules, 24(17), 3196. https://doi.org/10.3390/molecules24173196