Protolysis and Complex Formation of Organophosphorus Compounds—Characterization by NMR-Controlled Titrations
Abstract
:1. Introduction
1.1. Developing Technical Setups for Automated NMR Titrations
1.2. Some Comments on Macroscopic Protolytic Equilibria—Dissociation and Stability Constants
2. Results and Discussion
2.1. Phosphonic Acids
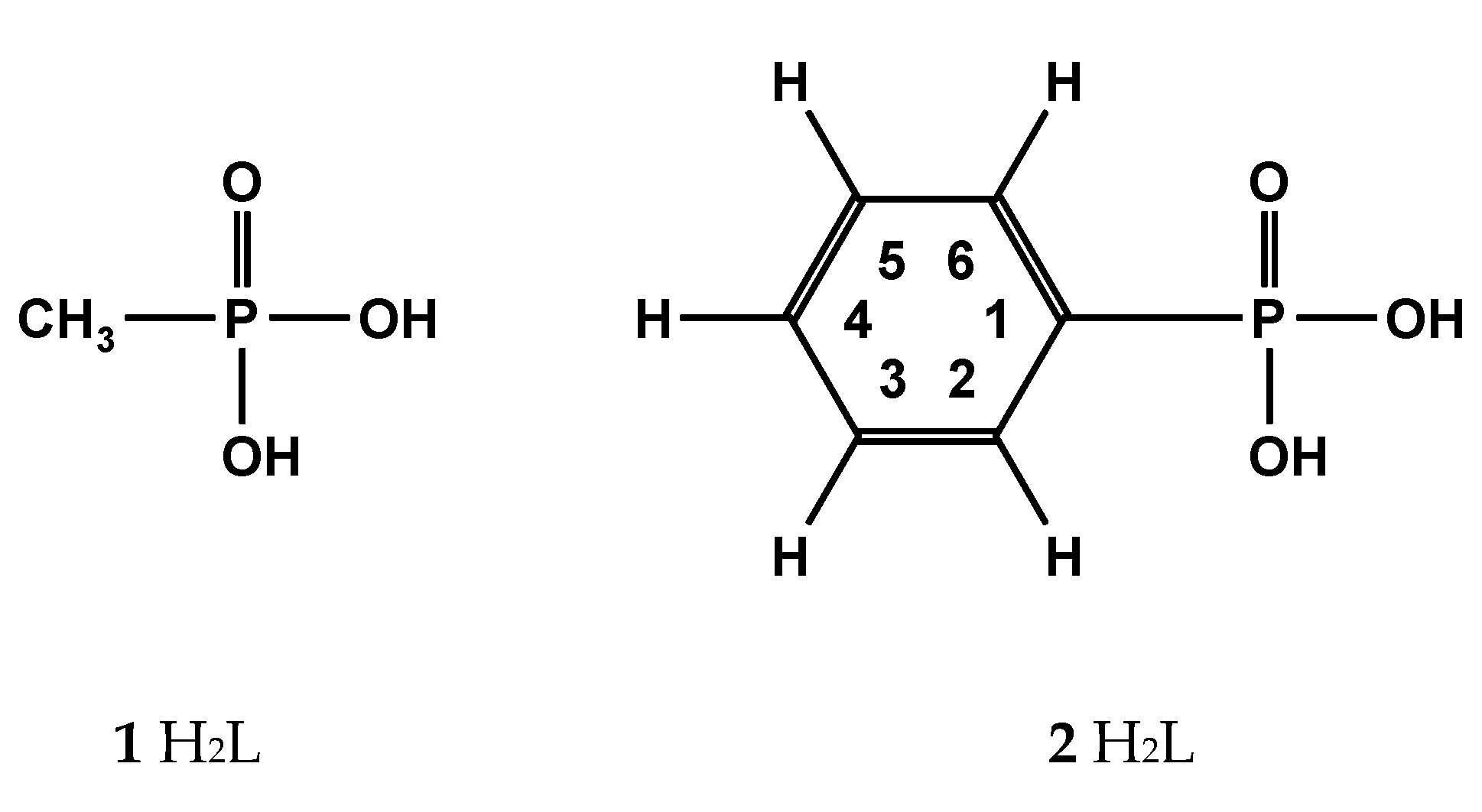
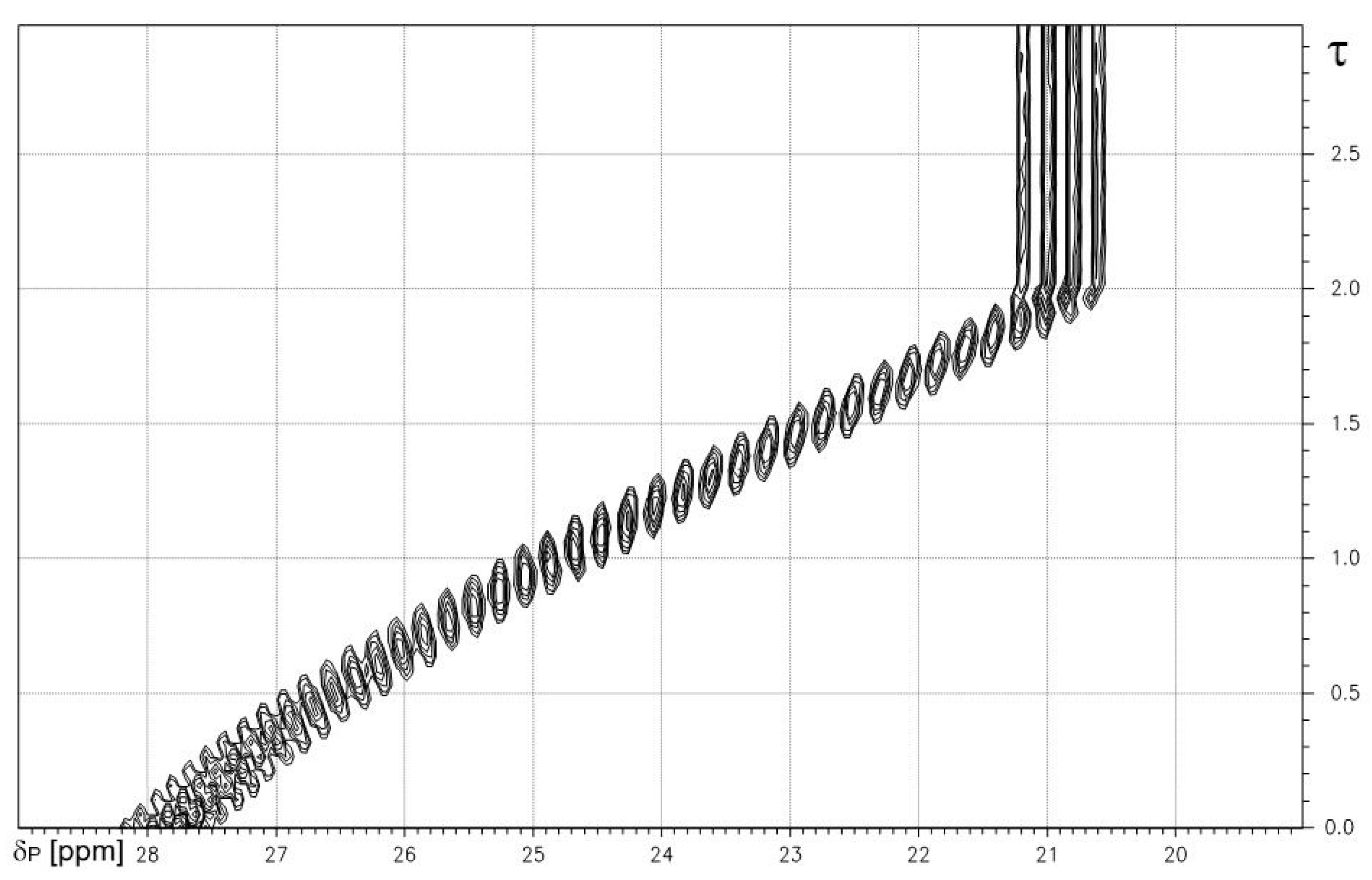
2.1.1. Methanephosphonic Acid 1
2.1.2. Phenylphosphonic Acid 2
2.2. Comparison of Aliphatic and Aromatic Aminophosphonic Acids
2.2.1. Aliphatic Aminophosphonic Acids
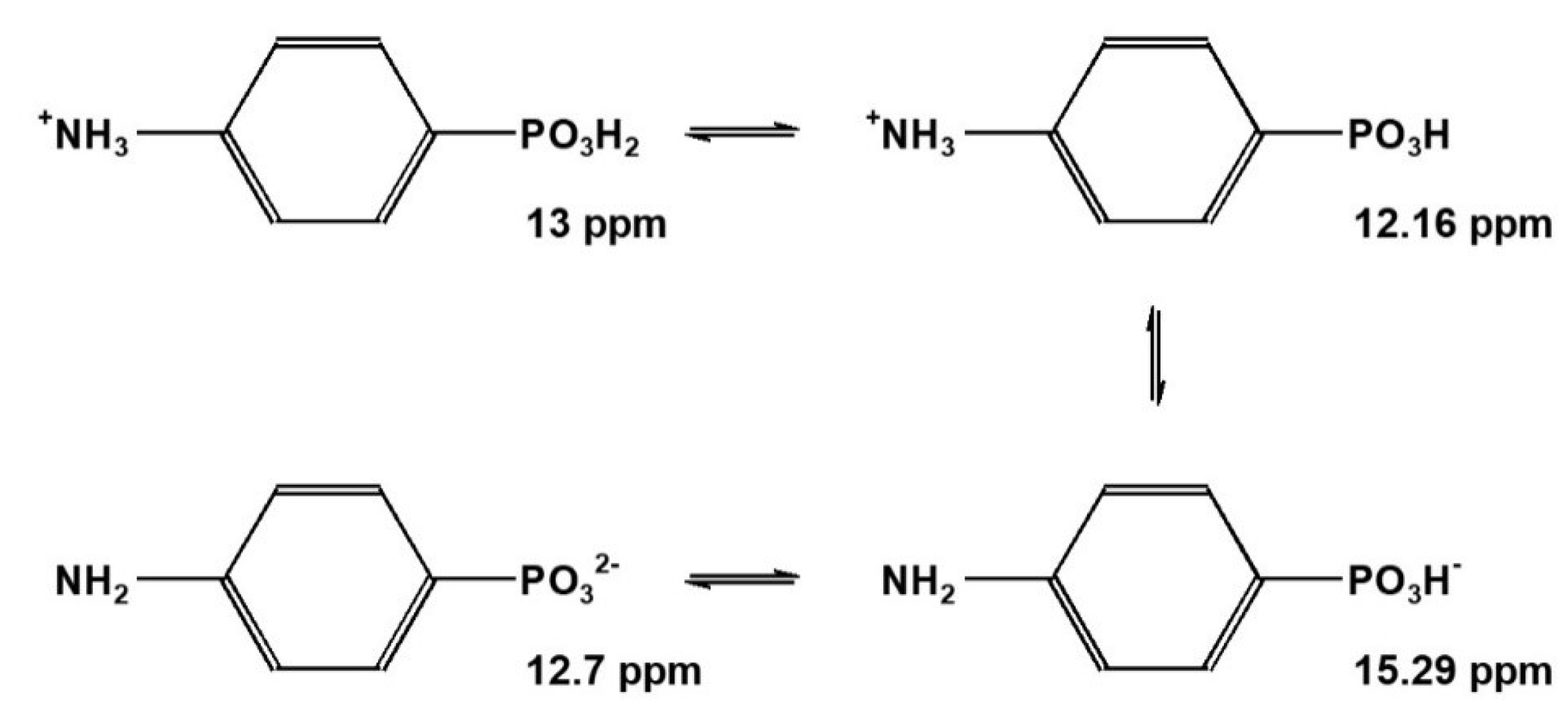
2.2.2. Aromatic p-Aminophenylphosphonic Acid 5
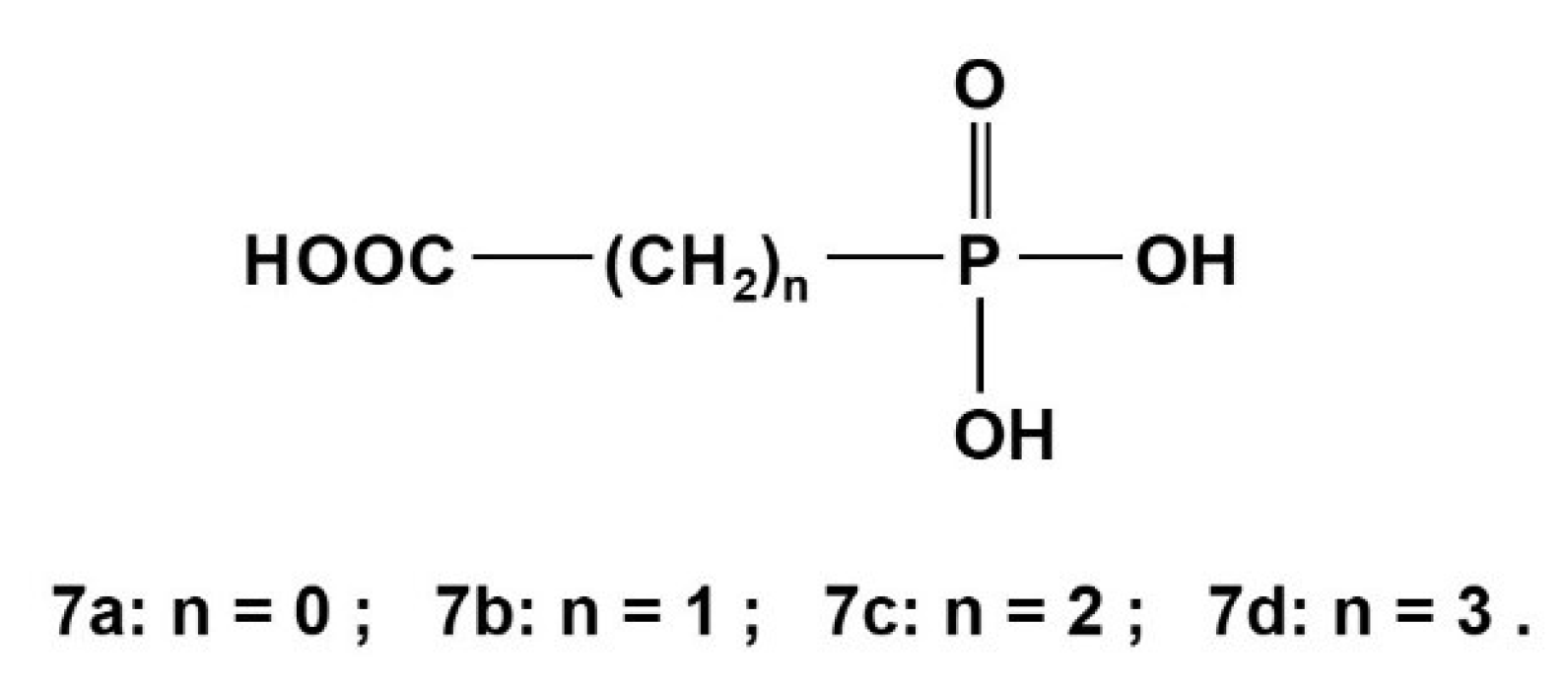
2.3. Phosphonocarboxylic Acids HOOC-(CH2)n-PO3H2 7a to 7d (n = 0 to 3)
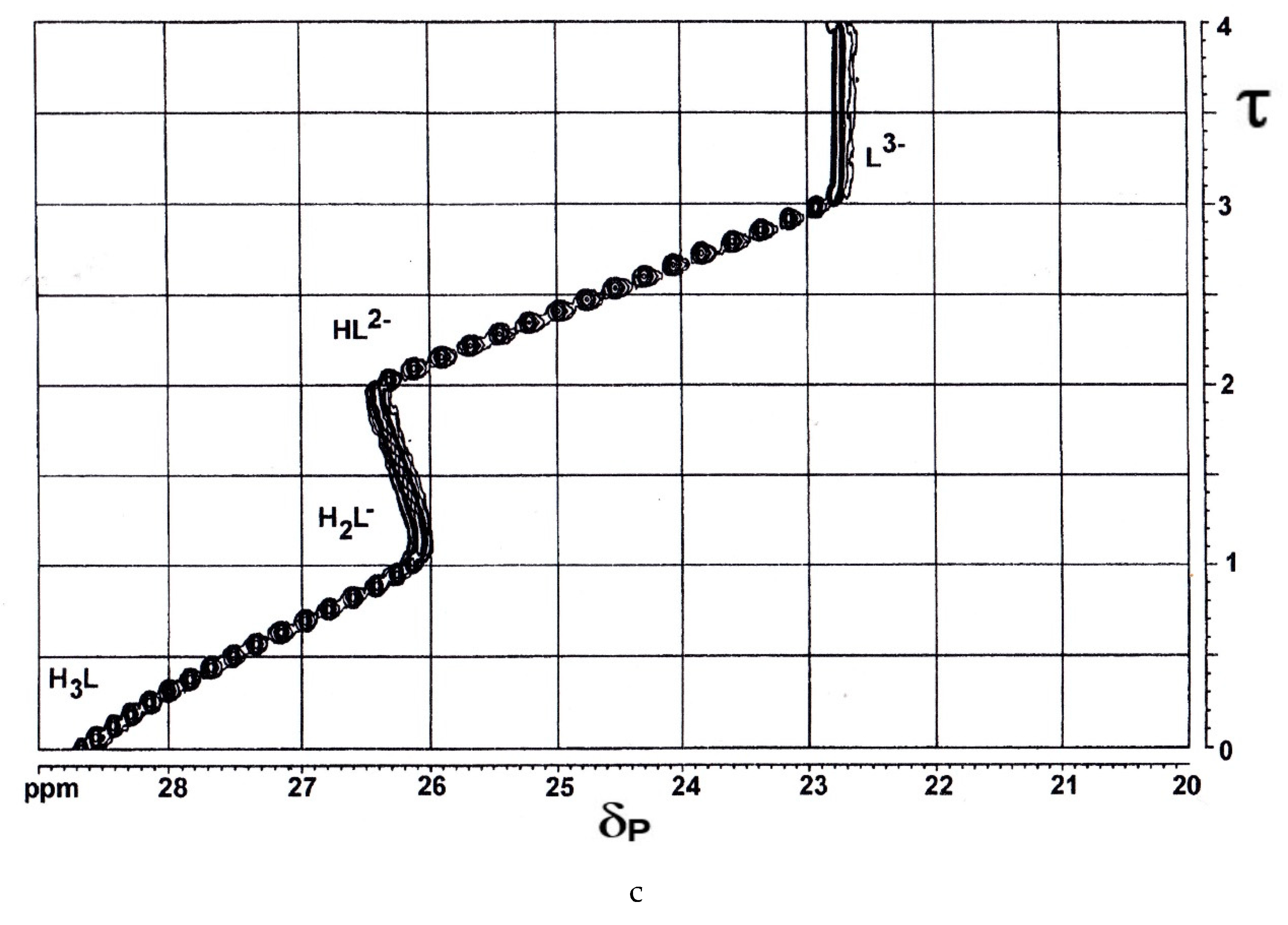
2.3.1. Compound 7c: 13C{1H}-NMR-Controlled Titration of 3-Phosphonopropionic Acid HOOC-CH2-CH2-PO3H2 7c.
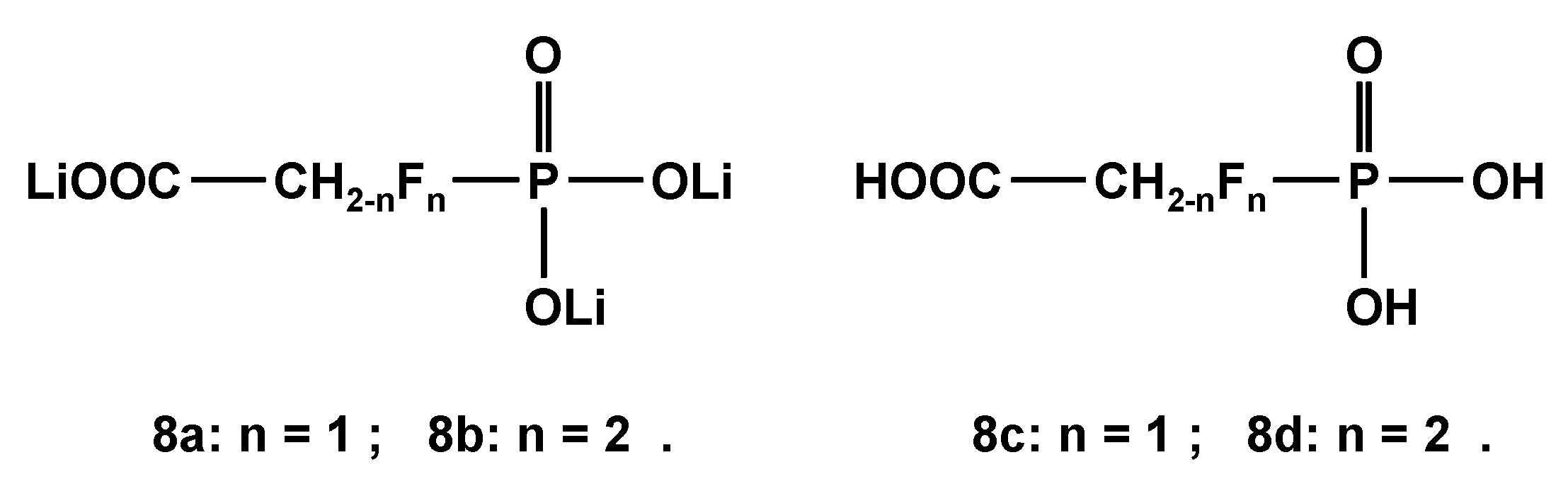
2.3.2. 19. F-NMR-Controlled Retro Titrations of Lithium Salts LiOOC-CH2-nFn-PO3Li2 8a and 8b
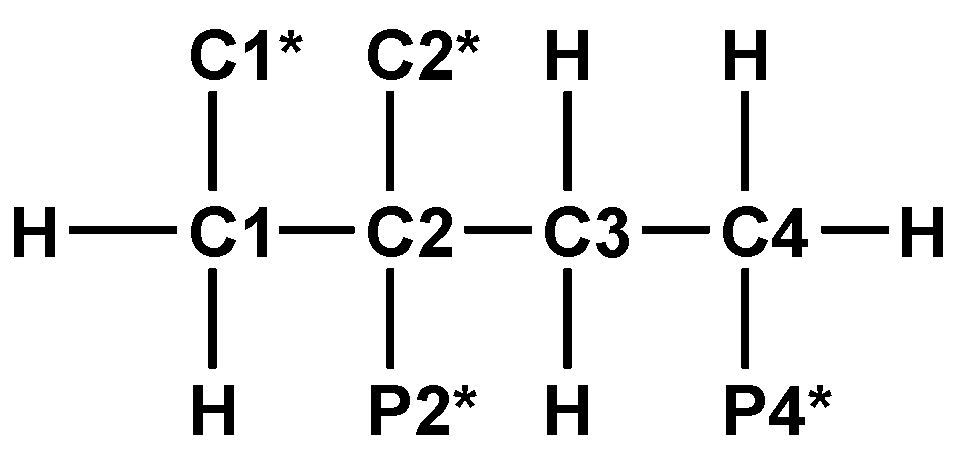
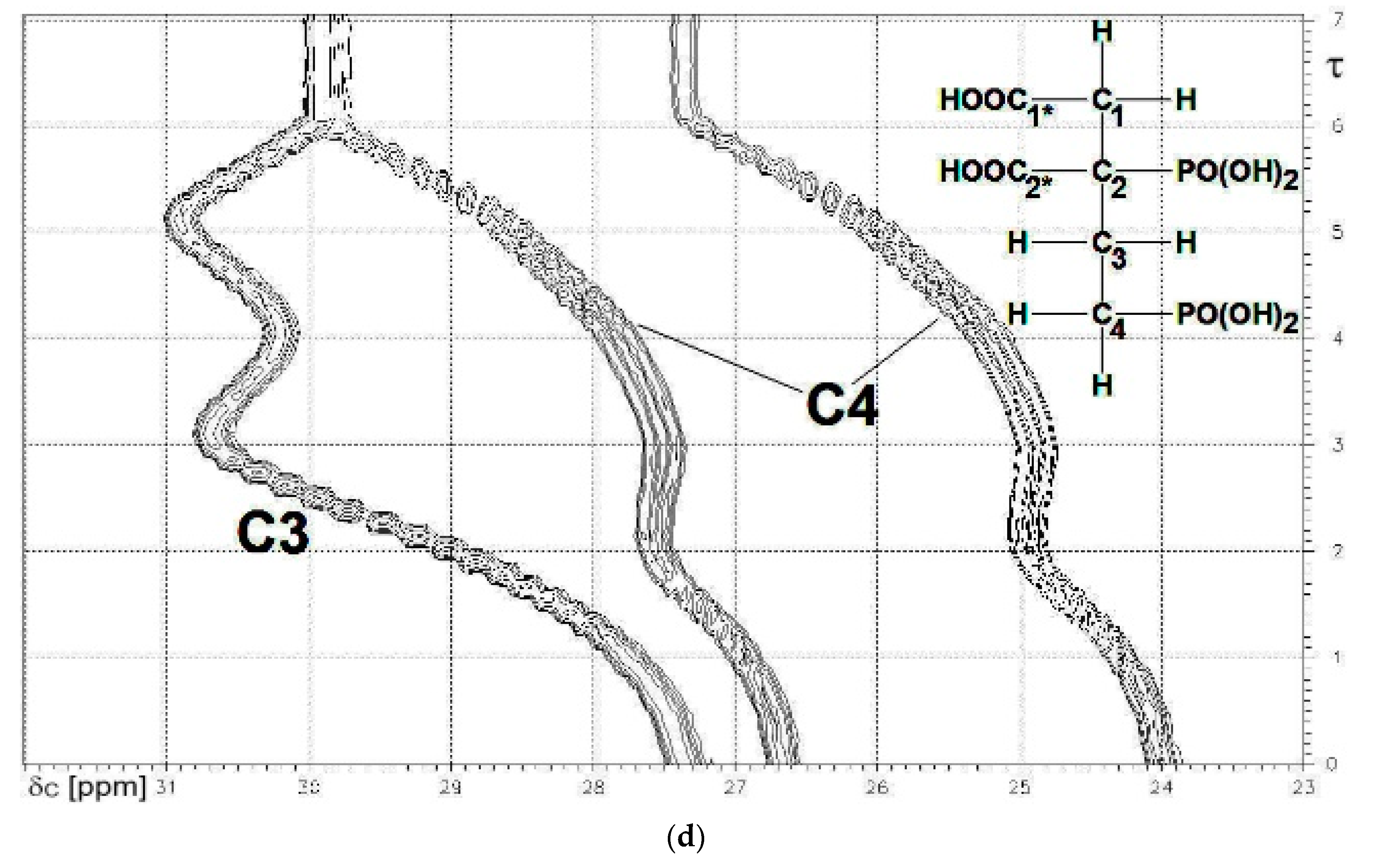
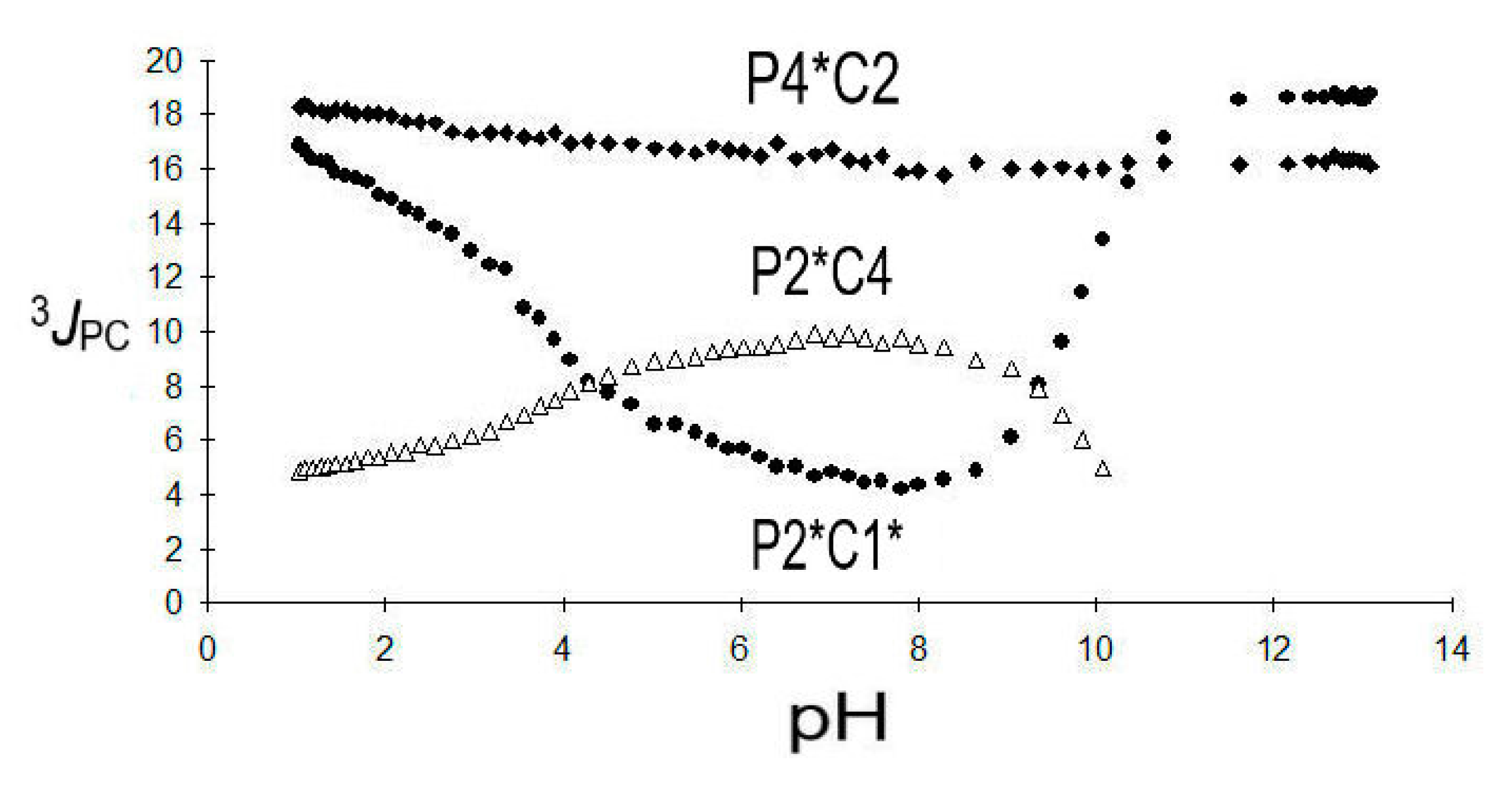
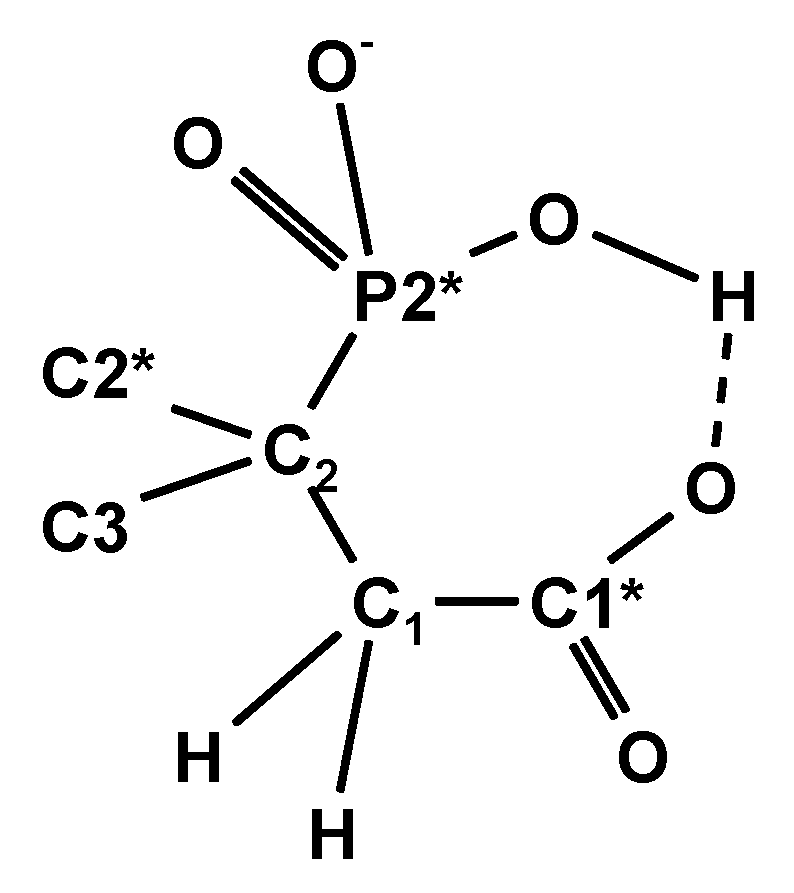
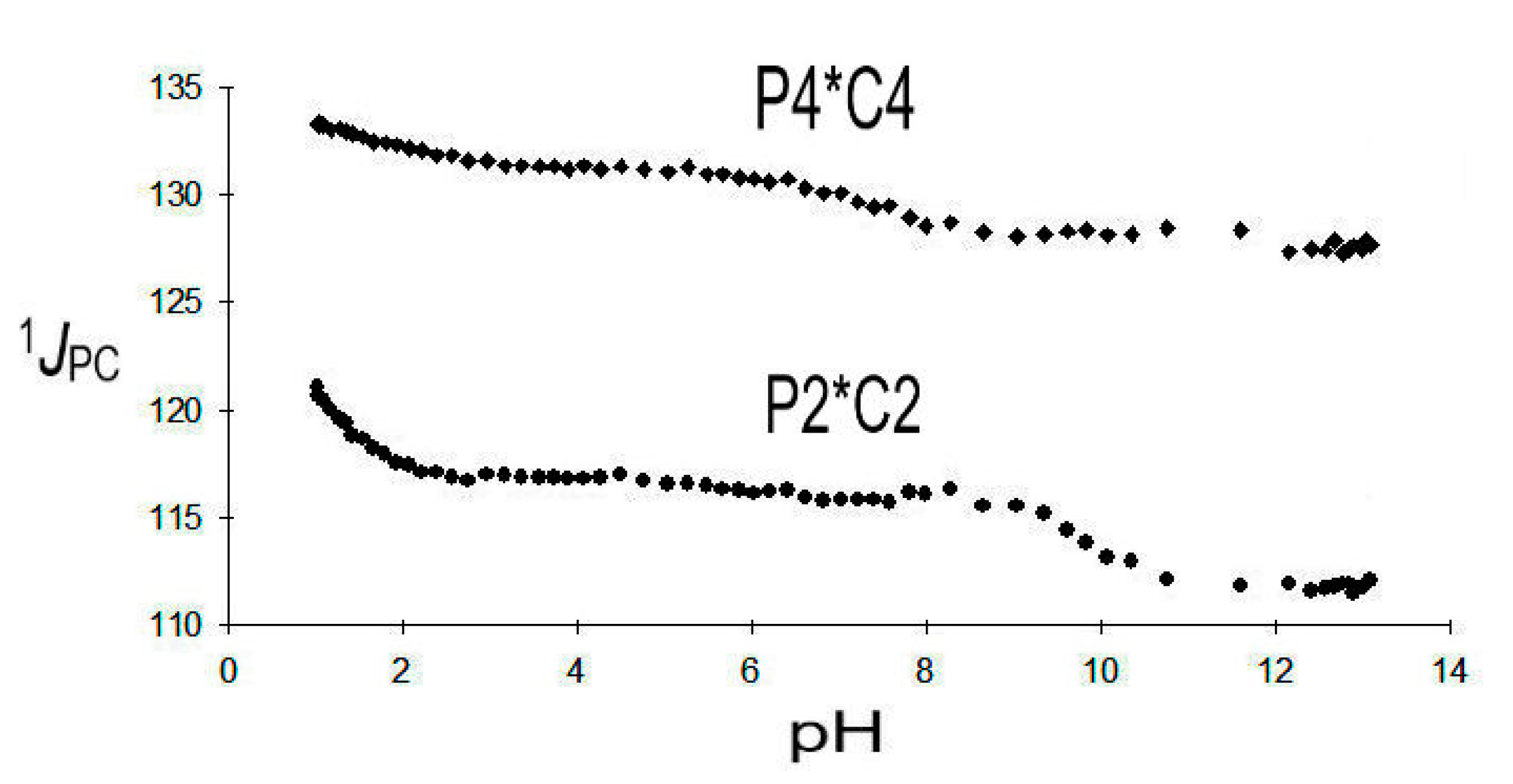
2.3.3. 2,4-Diphosphonobutane-1,2-Dicarboxylic Acid (DPBDC) 9
Some Comments on DPBDC 9
3. Conclusions
4. Experimental
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Van Wazer, J.R.; Callis, C.F.; Shoolery, J.N.; Jones, R.C. Principles of Phosphorus Chemistry. II. Nuclear Magnetic Resonance Measurements. J. Am. Chem. Soc. 1956, 78, 5715–5726. [Google Scholar] [CrossRef]
- Crutchfield, M.M.; Callis, C.F.; Irani, R.R.; Roth, G.C. Phosphorus Nuclear Magnetic Resonance Studies of Ortho and Condensed Phosphate. Inorg. Chem. 1962, 1, 813–817. [Google Scholar] [CrossRef]
- Hagen, R.; Roberts, J.D. Nuclear Magnetic Resonance Spectroscopy. 13C Spectra of Aliphatic Carboxylic Acids and Carboxylate Anions. J. Am. Chem. Soc. 1969, 91, 4504–4514. [Google Scholar] [CrossRef]
- Heubel, P.-H.; Popov, A.I.J. Acid Properties of Some Phosphonocarboxylic Acids. Sol. Chem. 1979, 8, 615–625. [Google Scholar] [CrossRef]
- Appleton, T.G.; Hall, R.J.; Harris, A.D.; Kimlin, H.A.; McMahon, I.J.N.M.R. Study of Acid-Base Equilibria of Aminoalkylphosphonic Acids, +NH3(CH2)nPO3H− (n = 1, 2, 3); Evidence for Cyclization in Solution. Austr. J. Chem. 1984, 37, 1833–1840. [Google Scholar] [CrossRef]
- Appleton, T.G.; Hall, R.J.; McMahon, I.J. NMR Spectra of Iminobis(methylenebisphosphonic acid), HN(CH2PO3H2)2 and Related Ligands and of Their Complexes with Platinum(II). Inorg. Chem. 1985, 25, 726–734. [Google Scholar] [CrossRef]
- Sawada, K.; Araki, T.; Suzuki, T. Complex Formation of Amino Polyphosphonates. 1. Potentiometric and Nuclear Magnetic Resonance Studies of Nitrilotris(methylenephosphonate) Complexes of the Alkaline Earth-Metal Ions. Inorg. Chem. 1987, 26, 1199–1204. [Google Scholar] [CrossRef]
- Sawada, K.; Araki, T.; Suzuki, T.; Doi, K. Complex Formation of Amino Polyphosphonates. 1. Stability and Structure of Nitrilotris(methylenephosphonate) Complexes of the Divalent Transitions-Metal Ions in Aqueous Solution. Inorg. Chem. 1989, 28, 2687–2688. [Google Scholar] [CrossRef]
- Sawada, K.; Kanda, T.; Naganuma, Y.; Suzuki, T. Formation and Protonation of Aminopolyphosphonate Complexes of Alkaline-earth and Divalent Transition-metal Ions in Aqueous Solution. J. Chem. Soc. Dalton Trans. 1993, 17, 2558–2562. [Google Scholar] [CrossRef]
- Sawada, K.; Myagawa, T.; Sakaguchi, T.; Doi, K. Structure and Thermodynamic Properties of Aminopolyphosphonate Complexes of the Alkaline-earth Metal Ions. J. Chem. Soc. Dalton Trans. 1993, 24, 3777–3784. [Google Scholar] [CrossRef]
- Sawada, K.; Ichikawa, T.; Uehara, K. Eight-membered chelate-ring complexes of cobalt(III)-polyamine complexes of aminopolyphosphonates in aqueous solution. J. Chem. Soc. Dalton Trans. 1996, 14, 3077–3085. [Google Scholar] [CrossRef]
- Matczak-Jon, E.; Kurzak, B.; Kamecka, A.; Sawka-Dobrowolska, W.; Kafarski, P.; Lejjczak, B. Zink(II) complexes of phosphonic acid analogues of glutamic acid. J. Chem. Soc. Dalton Trans. 1996, 3455–3464. [Google Scholar] [CrossRef]
- Matczak-Jon, E.; Kurzak, B.; Kamecka, A.; Sawka-Dobrowolska, W.; Kafarski, P. Interactions of zinc(II), magnesium(II) and calcium(II) with iminodimethylenediphosphonic acids in aqueous solutions. J. Chem. Soc. Dalton Trans. 1999, 20, 3627–3637. [Google Scholar] [CrossRef]
- Rohovec, J.; Kývala, M.; Vojtišek, P.; Hermann, P.; Lukeš, I. Synthesis, Crystal Structures, and Solution Properties of N-Methylene(phenyl)phosphinic Acid Derivatives of Cyclen and Cyclam. J. Inorg. Chem. 2000, 195–203. [Google Scholar] [CrossRef]
- Popov, K.; Popov, A.; Rönkkömäki, H.; Lajunen, L.H.J.; Hannu-Kuure, M.; Vendilo, A.; Tsirul’nikova, N. 31P, 23Na and 133Cs NMR equilibrium study of iminobis(methylenephosphonic acid) complexes with alkali metals. Inorg. Chimica Acta 2002, 344, 1–6. [Google Scholar] [CrossRef]
- Popov, A.; Rönkkömäki, H.; Popov, K.; Lajunen, L.H.J.; Vendilo, A. 31P NMR protonation equilibria study of iminobis(methylenphosphonic) acid and its derivatives at high pH. Inorg. Chimica Acta 2003, 353, 1–7. [Google Scholar] [CrossRef]
- Ohms, G.; Grossmann, G. Über die pH-Abhängigkeit der 31P- und 13C-NMR-Spektren von Cyclohexan-, Cyclohexen- und Benzenphosphonsäuren. Z. Anorg. Allg. Chem. 1987, 544, 232–240. [Google Scholar] [CrossRef]
- Rönkömäki, H.; Jokisaari, H.; Lajunen, L.H. 31P NMR and Potentiometric Studies on the Protonation of Isopropyl Esters of Chlodronic Acid. Acta Chem. Scand. 1993, 47, 331–337. [Google Scholar] [CrossRef]
- Popov, K.; Niskanen, E.; Rönkkömäki, H.; Lajunen, L.H.J. 31P NMR Study of organophosphonate protonation equilibrium at high pH. New J. Chem. 1999, 23, 1209–1213. [Google Scholar] [CrossRef]
- Szakács, Z.; Hägele, G. Accurate determination of low pK values by 1H NMR titration. Talanta 2004, 62, 819–825. [Google Scholar] [CrossRef]
- Szakács, Z.; Hägele, G.; Tyka, R. 1H/31P NMR pH indicator series to eliminate the glass electrode in NMR spectroscopic pKa determinations. Anal. Chim. Acta 2004, 522, 247–258. [Google Scholar] [CrossRef]
- Popov, K.; Rönkkömäki, H.; Lajunen, L.H. Guidelines for The NMR Measurements for Determination of High And Low pKa Values. Pure Appl. Chem. 2006, 78, 663–675. [Google Scholar] [CrossRef]
- Yesinowski, J.P.; Sunberg, R.J.; Benedict, J.J. pH Control and Rapid Mixing in Spinning NMR Samples. J. Magn. Res. 1982, 47, 85–90. [Google Scholar] [CrossRef]
- Glaser, J.; Henriksson, U.; Klason, T. A 205 TL NMR Titration Study of the Complex Formation between Tl(I) and Cl- in Aqueous Solution. Acta Chem. Scand. 1986, A40, 344–349. [Google Scholar] [CrossRef]
- Li, W. Gravity-driven pH adjustment for site-specific protein pKa measurement by solution-state NMR. Meas. Sci. Technol. 2017, 28. [Google Scholar] [CrossRef]
- Hägele, G.; Grzonka, M.; Kropp, H.-W.; Ollig, J.; Spiegl, H. Phosphonic and phosphinic acids: Monitoring protolytic and complex formation equilibria by titration dependent stopped-flow-NMR-techniques. Phosphorus Sulfur Silicon 1993, 77, 85–88. [Google Scholar] [CrossRef]
- Hägele, G.; Varbanov, S.; Ollig, J.; Kropp, H.-W. Aminomethylphosphine Oxides: Synthesis, Dissociation and Stability Constants, 31P{1H}-NMR-controlled Titration. Z. Anorg. Allg. Chemie 1994, 620, 914–920. [Google Scholar] [CrossRef]
- Hägele, G. 31P NMR controlled titrations of Phosphorus-Containing Acids and Bases in Protolysis and Complex Formation. In Phosphorus-31 NMR Spectral Properties in Compound Characterization and Structural Analysis; Quin, L.D., Verkade, J.G., Eds.; VCH Publishers: New York, NJ, USA, 1994; Chapter 30; pp. 395–409. [Google Scholar]
- Hägele, G.; Ollig, J. NMR-controlled titrations of phosphorus containing acids and bases. Comput. Chem. 1995, 19, 287–294. [Google Scholar]
- Ahrendt, C.; Hägele, G. The Photo-T-Concept: Hard- and software combination for the determination of macroscopic and microscopic dissociation constants. Comput. Chem. 1995, 19, 263–268. [Google Scholar] [CrossRef]
- Hägele, G.; Szakács, Z.; Ollig, J.; Hermens, S.; Pfaff, C. NMR-Controlled Titrations: Characterizing Aminophosphonates and Related Structures. Heteroat. Chem. 2000, 11, 562–582. [Google Scholar] [CrossRef]
- Hägele, G.; Grzonka, M.; Peters, J.; Spiegl, H.; Kropp, H.W.; Ollig, J.; Hermens, S.; Augner, S.; Uhlemann, C.; Pfaff, C.; et al. NMR-Controlled Titrations—Principles and Progress: Monitoring Protonation and Complex Formation Equilibria in Aqueous Solutions. Available online: https://www.theresonance.com/nmr-controlled-titration-download-the-paper/ (accessed on 2 September 2019).
- Pfaff, C.G. Softwareentwicklung zur Auswertung und Visualisierung Kernresonanzspektroskopisch Kontrollierter Titrationen. Ph.D. Thesis, Heinrich-Heine-Universität, Düsseldorf, Germany, 2002. [Google Scholar]
- Peters, M.; Siegfried, L.; Kaden, T.A. pH-metric and NMR studies of complexation of Zn2+, Cd2+ and Pb2+ with diazacrown ethers having dangling phosphonate groups. J. Chem. Soc. Dalton Trans. 2000, 24, 4664–4668. [Google Scholar] [CrossRef]
- Rabenstein, D.L.; Sayer, T.L. Determination of Microscopic Acid Dissociation Constants by Nuclear Magnetic Resonance Spectrometry. Anal. Chem. 1976, 48, 1141–1146. [Google Scholar] [CrossRef]
- Szakacs, Z.; Kraszni, M.; Noszal, B. Determination of microscopic acid-base parameters from NMR-pH titrations. Anal. Bioanal. Chem. 2004, 378, 1428–1448. [Google Scholar] [CrossRef]
- Szakacs, Z. NMR-Titrationen und neue Auswertekonzepte zur Aufklärung der Mikroskopischen Dissoziation und der pH-abhängigen Konformation von Biorelevanten Phophinsäuren und Carnosinderivaten. Ph.D. Thesis, Heinrich-Heine-Universität, Düsseldorf, Germany, 2002. [Google Scholar]
- Kývala, M.; Lukeš, I. Chemometrics 95. In Book of Abstracts, Proceedings of the 4th International Chemometrics Conference of the Czech Chemical Society, Pardubice, Czech Republic, 3–7 July 1995; University of Pardubice: Pardubice, Czech Republic, 1995; p. 63. [Google Scholar]
- Augner, S.; Kehler, J.; Szakács, Z.; Breuer, E.; Hägele, G. Ring-chain tautomerism and protolytic equilibria of 3-hydroxy-3-phosphonoisobenzo-furanone studied by 1H-, 13C-, and 31P-NMR-controlled titrations. New J. Chem. 2008, 32, 1608–1616. [Google Scholar] [CrossRef]
- Augner, S. Neue Entwicklungen zur Durchführung automatisierter NMR-Kontrollierter Titrationen von Phosphon- und Phosphinsäuren. Ph.D. Thesis, Heinrich-Heine-Universität, Düsseldorf, Germany, 2002. [Google Scholar]
- Kropp, H.-W.; Hägele, G. Unpublished results from my student Kropp. Interested readers may contact G. Hägele. Unpublished work. 1994. [Google Scholar]
- Lindner, A. 1-Phosphonopropan-1,2,3-tricarbonsäure—NMR- und Konformationsanalytische Untersuchungen. Ph.D. Thesis, Heinrich-Heine-Universität, Düsseldorf, Germany, 2000. [Google Scholar]
- Hägele, G. NMR-controlled titrations characterizing organophosphorus compounds. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 361–363. [Google Scholar]
- Ollig, J. Untersuchungen zur Titrationsabhängigen Kernresonanzspektroskopie. Ph.D. Thesis, Heinrich-Heine-Universität, Düsseldorf, Germany, 1996. [Google Scholar]
- Robitaille, P.-M.L.; Robitaille, P.A.; Brown, G.G., Jr.; Brown, G.G. An Analysis of the pH-Dependent Chemical-Shift Behavior of Phosphorus-Containing Metabolites. J. Magn. Res. 1991, 91, 73–84. [Google Scholar] [CrossRef]
- Popov, K.; Rönkkömäki, H.; Lajunen, L.J.J. Critical evaluation of stability constants of phosphonic acids. Pure Appl. Chem. 2001, 73, 1641–1677. [Google Scholar] [CrossRef]
- Clark, T.; Alex, A.; Beck, B.; Chandrasekhar, J.; Gedeck, P.; Horn, A.H.C.; Hutter, M.; Martin, B.; Rauhut, G.; Sauer, W.; et al. VAMP 4.4; Universität Erlangen-Nürnberg: Erlangen, Germany, 1990. [Google Scholar]
- Wozniak, M.; Nowogrocky, G. Acidites et complexes des acides (alkyl- et aminoalkyl-) phosphoniques—I: Determination potentiometrique des constantes d’acidite par affinement multiparametrique: Prise en compte de l’impurete carbonate. Talanta 1979, 26, 1135–1141. [Google Scholar] [CrossRef]
- Mohan, M.S.; Abbott, E.H. Metal complexes of biologically occurring aminophosphonic acids. J. Coord. Chem. 1978, 8, 175–182. [Google Scholar] [CrossRef]
- Kiss, T.; Balla, J.; Nagy, G.; Kozzlowski, H.; Kowalik, J. Complexes of aminophosphonates. I. Transition metal complexes of aminophosphonic acid analogues of α-alanine, β-alanine, phenylalanine and tyrosine. Inorg. Chim. Acta 1987, 138, 25–30. [Google Scholar] [CrossRef]
- Kropp, H.-W.; Hägele, G. Analytische und NMR-spektroskopische Untersuchungen an Organophosphorsäuren. Unpublished work.
- Burton, D.J.; Sprague, L.G.; Pietrzyk, D.J.; Edelmuth, S.H. A Facile Synthesis of Difluorophosphonoacetic Acid. J. Org. Chem. 1984, 49, 3437–3438. [Google Scholar] [CrossRef]
- Bier, A. Computereinsatz in der Analytischen Chemie zur Untersuchung von Protolyse- und Komplexbildungs-Gleichgewichten am Beispiel der Phosphonocarbonsäuren. Ph.D. Thesis, Heinrich-Heine-Universität, Düsseldorf, Germany, 1993. [Google Scholar]
- Rabenstein, D.L.; Sayer, T.L. Carbon-13 Chemical Shift parameters for Amines, Carboxylic Acids, and Amino Acids. J. Magn. Res. 1978, 24, 27–39. [Google Scholar] [CrossRef]







| Examples | NMR | Remarks |
|---|---|---|
| Phosphonic acids | ||
| CH3P(O)(OH)2 | 31P{1H} | |
| LiOOC-CH2-P(O)(OLi)2 | 31P{1H} | 1) |
| (HO)2(O)P-CH2-CH2-P(O)(OH)2 | 13C{1H} | |
| CH3-C(OH)[P(O)(OH)2]2, HEDP, etidronic acid | 31P{1H} | |
| NH2-CH2-CH2-C(OH)(P(O)(OH)2)2, pamidronic acid | 31P{1H} | |
| HOOC-CH2-CH(COOH)-CH(COOH)-P(O)(OH)2, PPTC | 31P{1H} | |
| Phosphinic acids | ||
| (CH3)2P(O)OH | 31P{1H} | |
| HOOC-CH2-CH2-P(CH3)(O)OH | 13C{1H}, 1H | 2) |
| HO(O)(CH3)P-CH2-CH2-P(CH3)(O)OH | 13C{1H} | |
| HO(O)(CH3)P-CH2-CH2-C(H)(NH2)COOH | 1H | 2, 3) |
| Carboxylic acids | ||
| CH3COOH | 13C{1H} | |
| CH(CH3)2-CH2-CH(NH2)-C(O)-NH-CH(CH3)-COOH, peptide Leu-Ala | 13C{1H} | |
| CH2=CF-CH2-C(CH3)(NH2)-COOH | 19F |
| 1 in H2O | ||||||
|---|---|---|---|---|---|---|
| Method | 13C{1H} | 31P{1H} | 31P | 31P{1H} | ||
| Exp. | a) | b) | c) | d) | ||
| Species | δC | 1JPC | δP | δP | 2JPH | δP |
| [ppm] | [Hz] | [ppm] | [ppm] | [Hz] | [ppm] | |
| H2L | 14.27 | 135.92 | 33.03 | 33.03 | −17.65 | 31.76 |
| HL− | 15.53 | 133.82 | 24.79 | 24.79 | −16.52 | 24.94 |
| L2− | 16.61 | 129.95 | 21.08 | 21.08 | −15.52 | 20.94 |
| Gradients | δC | 1JPC | δP | δP | 2JPH | δP |
| Δ1 | 1.26 | −2.10 | −8.24 | −8.24 | 1.13 | |
| Δ2 | 1.08 | −3.87 | −3.71 | −3.71 | 1.00 | |
| pKi | ||||||
| pK1 | 2.27 | 2.06 | 2.00 | 2.33 | ||
| pK2 | 7.85 | 7.66 | 7.68 | 7.78 | ||
| Phenylphosphonic Acid 2 | ||
|---|---|---|
| vs. TMAOH | vs. NaOH | |
| a) | b) | |
| δP(H2L) | 18.39 | 17.77 |
| δP(HL−) | 13.77 | 13.75 |
| δP(L2−) | 11.69 | 11.72 |
| Δ1 | −4.62 | −4.02 |
| Δ2 | −2.08 | −2.03 |
| pK1 | 1.74 | 1.86 |
| pK2 | 7.28 | 7.16 |
| δC and nJPC for Species | Gradients | ||||
|---|---|---|---|---|---|
| Parameters | H2L | HL− | L2− | Δ1 | Δ2 |
| δC C1 | 133.66 | 138.15 | 143.83 | +4.48 | +5.69 |
| δC C2/6 | 133.68 | 133.45 | 133.43 | −0.24 | −0.02 |
| δC C3/5 | 131.99 | 131.59 | 130.95 | −0.40 | −0.64 |
| δC C4 | 135.66 | 134.03 | 131.98 | −1.58 | −2.06 |
| 1JPC | 183.48 | 177.02 | 167.32 | −6.46 | −9.70 |
| 2JPC | 10.51 | 9.72 | 8.79 | −0.79 | −0.93 |
| 3JPC | 14.84 | 13.90 | 12.65 | −0.94 | −1.25 |
| 4JPC | 3.08 | 2.91 | 2.74 | −0.17 | −0.17 |
| Dissociation Species | ||
|---|---|---|
| Macroscopic | Microscopic | |
| H3L+ | +NH3-R-PO3H2 | |
| H2L | +NH3-R-PO3H− | NH2-R-PO3H2 |
| HL− | +NH3-R-PO32− | NH2-R-PO3H− |
| L2− | NH2-R-PO32− | |
| 3 | 4 | ||||||
|---|---|---|---|---|---|---|---|
| 13C{1H} a | 31P{1H} b | Pot. c | 13C{1H} d | 31P{1H} e | 31P{1H} f | Pot. g | |
| NaOH | NaOH | NaOH | NaOH | TMAOH | NaOH | TMAOH | |
| pK1 | 0.70 | 0.31 | 0.3 | 1.02 | 1.22 | 1.26 | 1.14 |
| pK2 | 5.72 | 5.63 | 5.58 | 6.38 | 6.23 | 6.24 | 6.34 |
| pK3 | 10.64 | 10.21 | 10.28 | 11.50 | 11.06 | 11.08 | 11.06 |
| 3 | 4 | 5 | ||
|---|---|---|---|---|
| 31P{1H} a | 31P{1H} b | 31P{1H} a | 31P{1H} c | |
| Species | δP | δP | δP | δP |
| H3L+ | 15 * | 22.9 | 23.4 | 13 * |
| H2L | 14.92 | 19.29 | 19.36 | 12.16 |
| HL− | 13.08 | 16.80 | 16.81 | 15.29 |
| L2− | 22.25 | 19.39 | 19.72 | 12.70 |
| Gradients | ||||
| Δ1 | −0.08 | −3.61 | −4.04 | −0.84 |
| Δ2 | −1.84 | −2.49 | −2.55 | +3.13 |
| Δ3 | +9.17 | +2.59 | +3.91 | −2.59 |
| 3 in H2O | 4 in H2O | |||||
|---|---|---|---|---|---|---|
| Species | δC(C1) | 1JPC | δC(C2) | δC(C1) | 1JPC | δC(C2) |
| H3L+ | 46.80 | 151.5 | 16.0 | 27.50 | 137.4 | 37.09 |
| H2L | 47.70 | 143.8 | 16.43 | 28.73 | 131.4 | 38.22 |
| HL− | 49.07 | 134.5 | 17.23 | 29.47 | 124.8 | 39.28 |
| L2− | 48.15 | 138.0 | 19.79 | 35.45 | 126.5 | 39.76 |
| Gradients | ||||||
| Δ1 | +0.9 | −8.7 | +0.43 | +1.23 | −6.0 | +1.13 |
| Δ2 | +1.37 | +9.3 | +0.80 | +0.74 | −6.6 | +1.04 |
| Δ3 | −0.92 | +3.5 | +2.55 | +5.98 | +1.3 | +0.47 |
| 5 | 2 | 6 | |
|---|---|---|---|
| pK1 | 0.44 | 1.88 | 4.68 |
| pK2 | 3.95 | 7.15 | |
| pK3 | 7.56 |
| HOOC-(CH2)n-PO3H2 | ||||||
|---|---|---|---|---|---|---|
| 7a | 7a [4] | 7b | 7c | 7c [4] | 7d | |
| n = 0 a | n = 0 | n = 1 b | n = 2 b | n = 2 | n = 3 b | |
| pK1 | 0.78 | 1.7 ± 0.1 | 1.22 ± 0.166 | 2.58 ± 0.013 | 2.26 ± 0.04 | 2.276 ± 0.006 |
| pK2 | 3.60 | 3.59 ± 0.02 | 4.942 ± 0.004 | 4.633 ± 0.004 | 4.63 ± 0.02 | 4.776 ± 0.004 |
| pK3 | 7.57 | 7.56 ± 0.02 | 8.099 ± 0.003 | 7.738 ± 0.003 | 7.75 ± 0.02 | 7.969 ± 0.003 |
| (a) | ||||||||||
| 7 | n | Species | δC(C1) | δC(C2) | δC(C3) | δC(C4) | 1JPC | 2JPC | 3JPC | 4JPC |
| a | 0 | H3L | 176.8 | 246.6 | ||||||
| H2L− | 178.7 | 236.7 | ||||||||
| HL2− | 181.8 | 231.8 | ||||||||
| L3− | 187.3 | 220.0 | ||||||||
| b | 1 | H3L | 172.91 | 37.68 | 128.6 | n. r. | ||||
| H2L− | 175.44 | 39.30 | 117.8 | n. r. | ||||||
| HL2− | 178.85 | 41.64 | 119.2 | n. r. | ||||||
| L3− | 181.74 | 43.50 | 112.6 | n. r. | ||||||
| c | 2 | H3L | 179.25 | 30.09 | 24.51 | 138.5 | 3.6 | 17.3 | ||
| H2L− | 180.68 | 31.40 | 25.91 | 135.1 | 3.2 | 17.8 | ||||
| HL2− | 185.01 | 34.27 | 27.50 | 133.0 | 4.1 | 18.7 | ||||
| L3− | 186.71 | 35.61 | 28.99 | 130.3 | 3.6 | 19.8 | ||||
| d | 3 | H3L | 180.38 | 36.67 | 20.44 | 28.19 | 135.2 | 4.0 | 17.4 | n. r. |
| H2L− | 181.12 | 37.46 | 21.58 | 29.74 | 133.5 | 3.8 | 17.2 | n. r. | ||
| HL2− | 185.86 | 41.45 | 23.13 | 30.46 | 132.5 | 3.9 | 17.7 | n. r. | ||
| L3− | 186.41 | 42.01 | 24.08 | 31.92 | 130.1 | 3.4 | 17.9 | n. r. | ||
| (b) | ||||||||||
| 7 | n | Gradients | δC(C1) | δC(C2) | δC(C3) | δC(C4) | 1JPC | 2JPC | 3JPC | |
| a | 0 | Δ1 | +1.9 | −9.9 | ||||||
| Δ2 | +3.1 | −4.9 | ||||||||
| Δ3 | +5.5 | −11.8 | ||||||||
| b | 1 | Δ1 | +2.53 | +1.62 | −10.8 | |||||
| Δ2 | +3.41 | +2.34 | +1.4 | |||||||
| Δ3 | +2.89 | +1.86 | −6.6 | |||||||
| c | 2 | Δ1 | +1.43 | +1.31 | +1.40 | −3.4 | −0.3 | 0.5 | ||
| Δ2 | +4.33 | +2.87 | +1.59 | −2.1 | 0.9 | 0.9 | ||||
| Δ3 | +1.70 | +1.34 | +1.49 | −2.7 | −0.5 | 1.1 | ||||
| d | 3 | Δ1 | +0.74 | +0.79 | +1.14 | 1.56 | −1.7 | −0.2 | −0.2 | |
| Δ2 | +4.74 | +3.99 | +1.55 | 0.72 | −1.0 | 0.1 | 0.5 | |||
| Δ3 | +0.55 | +0.56 | +0.95 | 1.46 | −2.4 | −0.5 | 0.2 | |||
| Shifts | δP | Error |
|---|---|---|
| H3L | 29.93 | ±0.25 |
| H2L− | 24.56 | ±0.02 |
| HL2− | 25.88 | ±0.01 |
| L3− | 22.06 | ±0.01 |
| Gradients | ||
| Δ1 | −5.37 | |
| Δ2 | +1.32 | |
| Δ3 | −3.82 |
| HOOC-CH2-nFn-PO3H2 | |||
|---|---|---|---|
| 8c n = 1 | 8d n = 2 | ||
| [44] | [44] | [52] | |
| pK1 | 1.05 | 0.52 | 1.30 |
| pK2 | 3.43 | 2.22 | 1.95 |
| pK3 | 7.08 | 6.36 | 6.16 |
| HOOC-CH2-nFn-PO3H2 | ||||
|---|---|---|---|---|
| 8c | 8d | 8c | 8d | |
| n = 1 | n = 2 | n = 1 | n = 2 | |
| 19F | 19F | |||
| Species | δF | δF | 2JPF | 2JPF |
| H3L | −38.27 | 44.24 | 67.8 | 76.5 |
| H2L− | −38.72 | 50.24 | 65.5 | 88.6 |
| HL2− | −29.31 | 53.05 | 70.3 | 92.8 |
| L3− | −27.15 | 55.24 | 63.4 | 82.0 |
| Gradients | ||||
| Δ1 | −0.45 | +6.00 | −2.3 | +12.1 |
| Δ2 | −9.41 | +2.81 | +4.8 | +4.2 |
| Δ3 | −8.16 | +2.19 | −6.9 | −10.8 |
| 13C{1H} NMR [44] | Potentiometric [44] | Potentiometric [53] | |
|---|---|---|---|
| pK1 | 1.07 | 0.6 | 1.806 ± 0.066 |
| pK2 | 2.73 | 2.42 | 2.250 ± 0.021 |
| pK3 | 4.82 | 4.32 | 4.078 ± 0.005 |
| pK4 | 7.05 | 6.46 | 6.562 ± 0.004 |
| pK5 | 8.95 | 8.18 | 8.664 ± 0.006 |
| pK6 | 11.62 | 10.75 | 12.839 ± 0.007 |
| CTitrand | 0.262 (DPBDC) * | 0.0050 (DPBDC) * | 0.01119 (DPBDC) * |
| CTitrator | 3.986 (NaOH) * | 0.0975 (NaOH) * | 0.09863 (TMAOH) * |
| CIon buffer | 0 | 0.1 (NaCl) * | 0.09863 (TMANO3) * |
| C1 | C2 | C3 | C4 | C1* | C2* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | δC | δC | 1JPC | 3JPC | δC | δC | 1JPC | 3JPC | δC | 3JPC | δC |
| H6L | 38.29 | 52.19 | 124.2 | 18.4 | 27.26 | 25.11 | 134.0 | 4.7 | 177.23 | 17.5 | 177.53 |
| H5L− | 38.70 | 52.42 | 116.8 | 18.2 | 27.51 | 25.56 | 132.4 | 5.1 | 177.82 | 15.9 | 178.66 |
| H4L2− | 39.38 | 52.98 | 116.9 | 17.3 | 29.09 | 26.34 | 131.3 | 6.3 | 178.85 | 12.5 | 179.18 |
| H3L3− | 41.78 | 54.06 | 116.7 | 16.8 | 30.95 | 26.15 | 131.3 | 9.0 | 181.69 | 6.4 | 180.70 |
| H2L4− | 43.28 | 54.66 | 115.9 | 16.6 | 29.98 | 26.54 | 130.3 | 9.9 | 183.46 | 4.3 | 181.53 |
| HL5− | 43.26 | 55.04 | 115.8 | 15.9 | 30.99 | 27.51 | 128.3 | 9.4 | 184.08 | 5.3 | 182.53 |
| L6− | 42.70 | 55.01 | 111.7 | 16.3 | 29.87 | 28.65 | 127.7 | - | 184.82 | 18.9 | 186.15 |
| Gradients | |||||||||||
| Δ1 | +0.41 | +0.23 | −7.4 | −0.2 | +0.25 | +0.45 | −1.6 | +0.4 | +0.59 | −1.6 | +1.13 |
| Δ2 | +0.68 | +0.56 | +0.1 | −0.9 | +1.58 | +0.78 | −1.1 | +1.2 | +1.03 | −3.4 | +0.52 |
| Δ3 | +1.40 | +1.08 | −0.2 | −0.5 | +1.86 | −0.19 | 0.0 | +2.7 | +2.84 | −6.1 | +1.52 |
| Δ4 | +1.50 | +0.60 | −0.8 | −0.2 | −0.97 | +0.39 | −1.0 | +0.9 | +1.77 | −2.1 | +0.83 |
| Δ5 | −0.02 | +0.38 | −0.1 | −0.7 | +1.01 | +0.97 | −2.0 | −0.5 | +0.62 | +1.0 | +1.00 |
| Δ6 | −0.56 | −0.03 | −4.1 | +0.4 | −1.12 | +1.14 | −0.6 | - | +0.74 | +13.6 | +3.62 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hägele, G. Protolysis and Complex Formation of Organophosphorus Compounds—Characterization by NMR-Controlled Titrations. Molecules 2019, 24, 3238. https://doi.org/10.3390/molecules24183238
Hägele G. Protolysis and Complex Formation of Organophosphorus Compounds—Characterization by NMR-Controlled Titrations. Molecules. 2019; 24(18):3238. https://doi.org/10.3390/molecules24183238
Chicago/Turabian StyleHägele, Gerhard. 2019. "Protolysis and Complex Formation of Organophosphorus Compounds—Characterization by NMR-Controlled Titrations" Molecules 24, no. 18: 3238. https://doi.org/10.3390/molecules24183238