Nanomaterials Based on Fe3O4 and Phthalocyanines Derived from Cashew Nut Shell Liquid
Abstract
:1. Introduction
2. Results
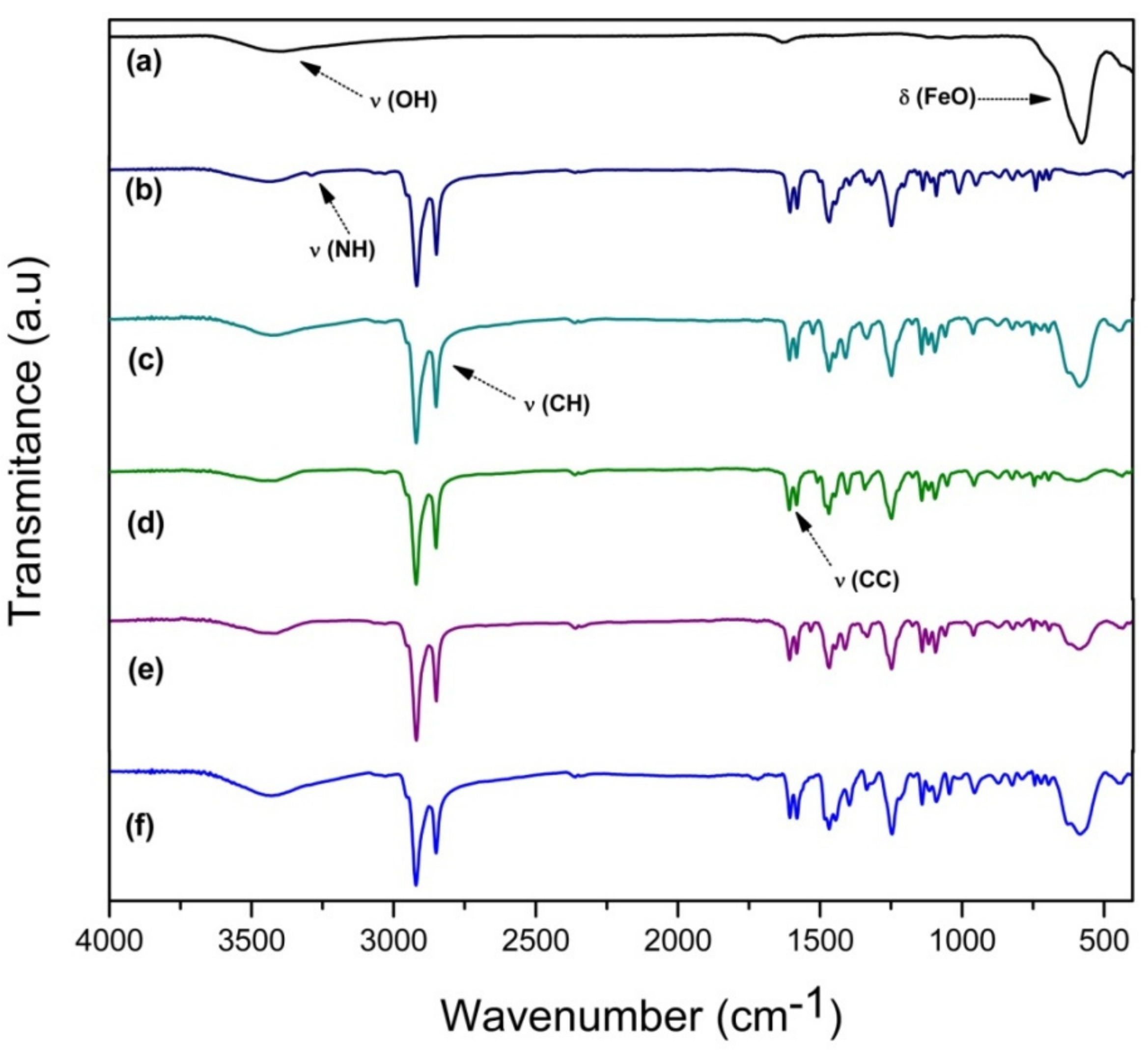
2.1. FT-IR Spectra
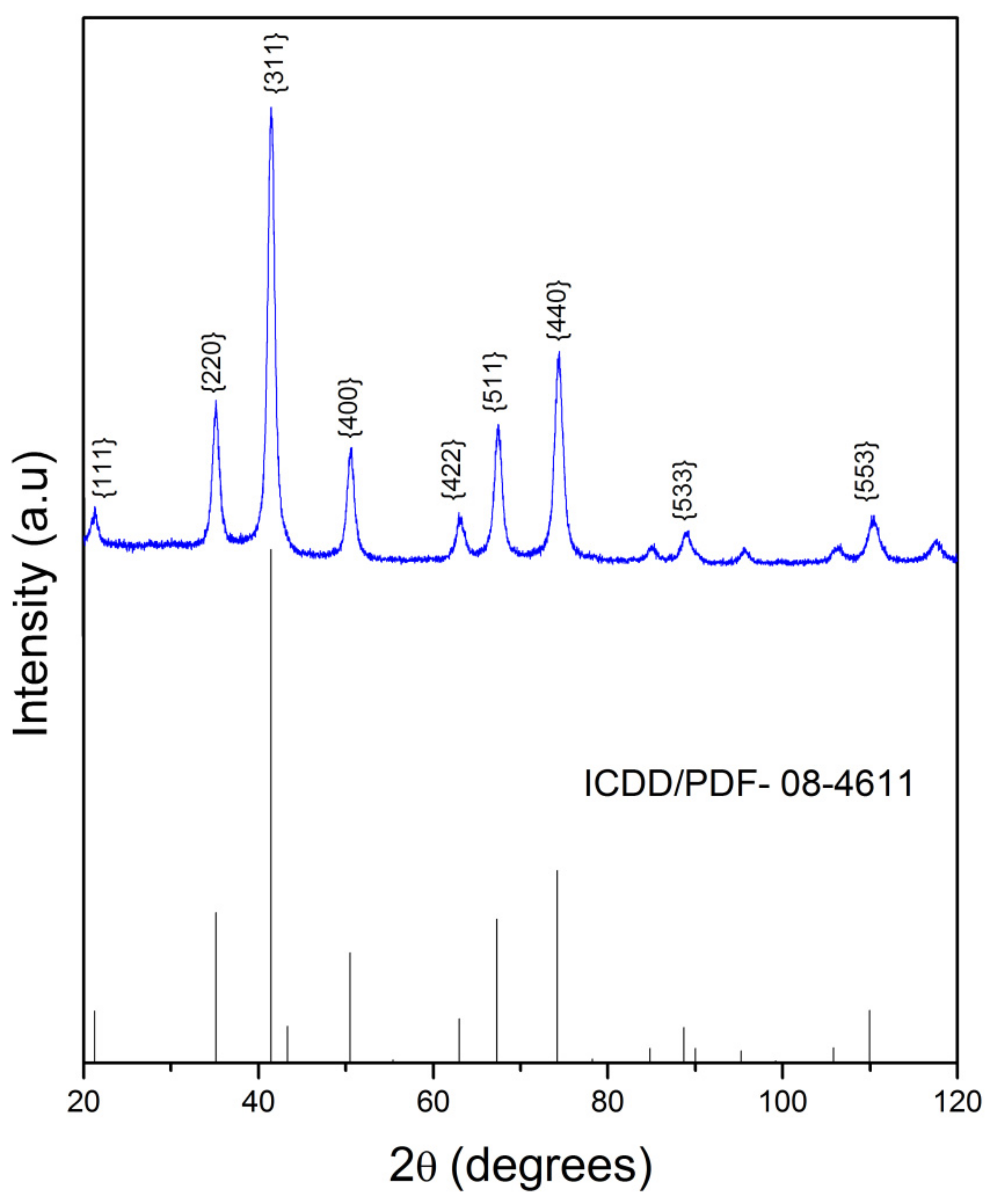
2.2. X-Ray Powder Diffraction
2.3. Thermal Analysis
2.4. Transmission Electron Microscopy
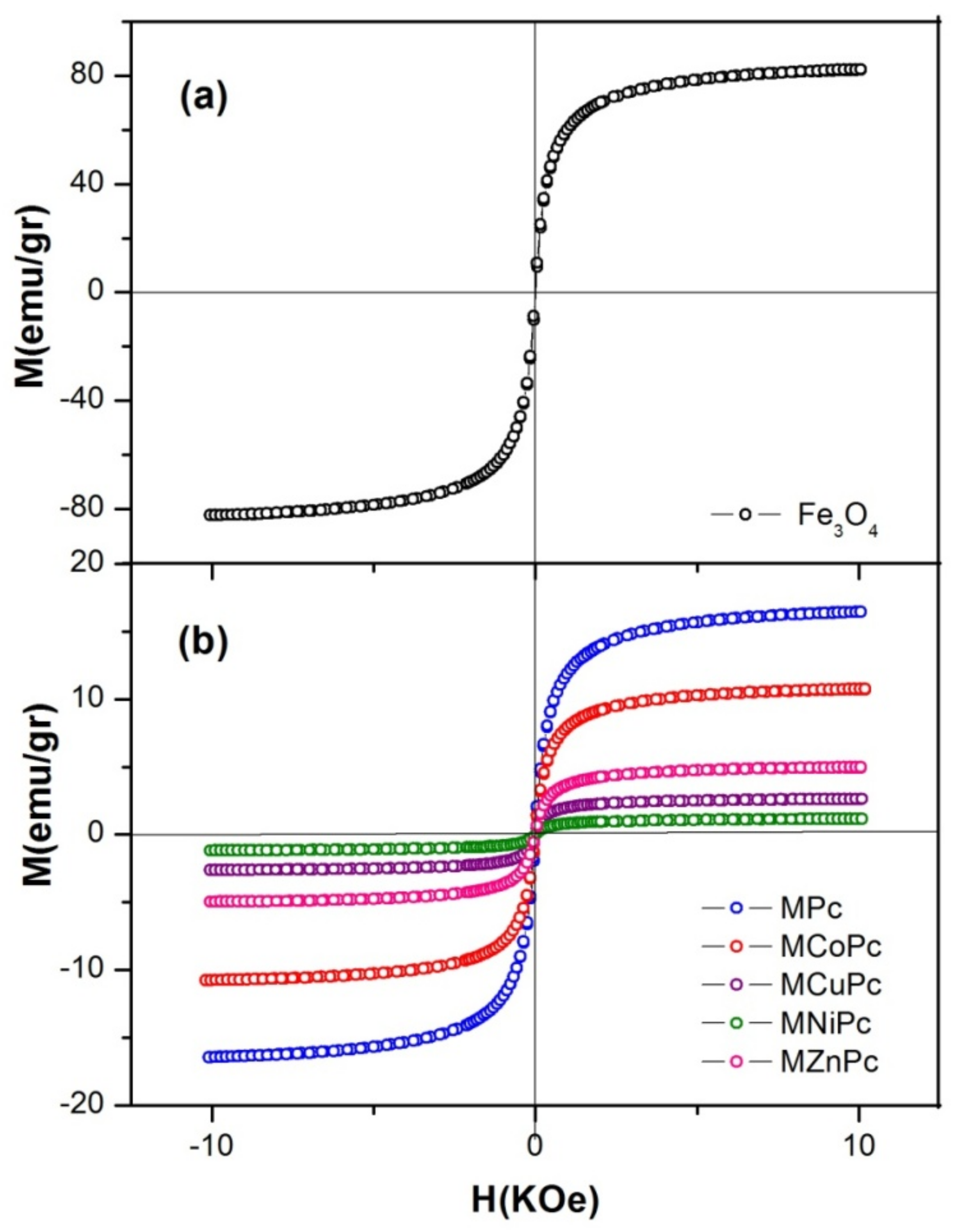
2.5. Magnetic Measurements
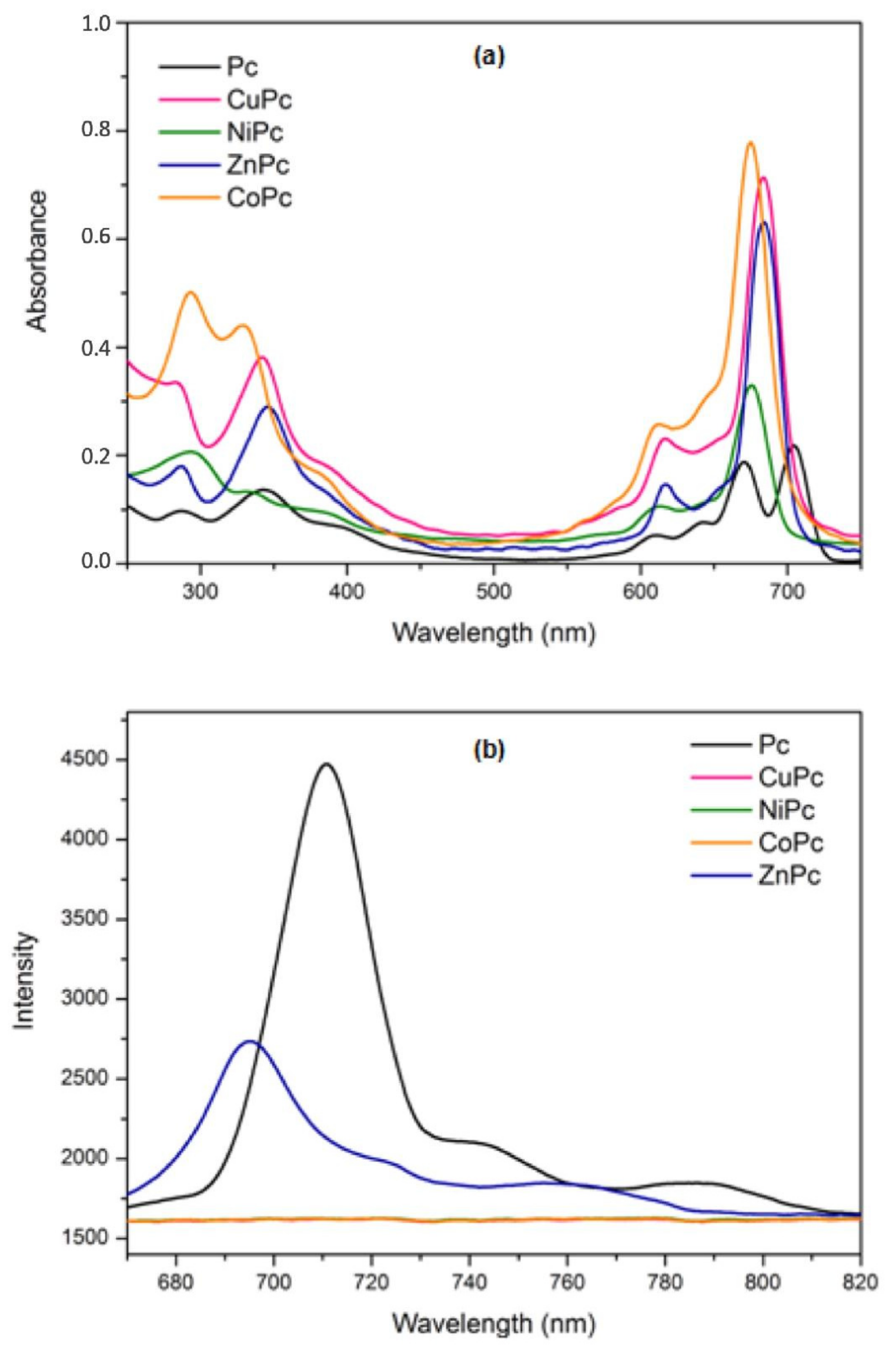
2.6. Optical Properties
3. Materials and Methods
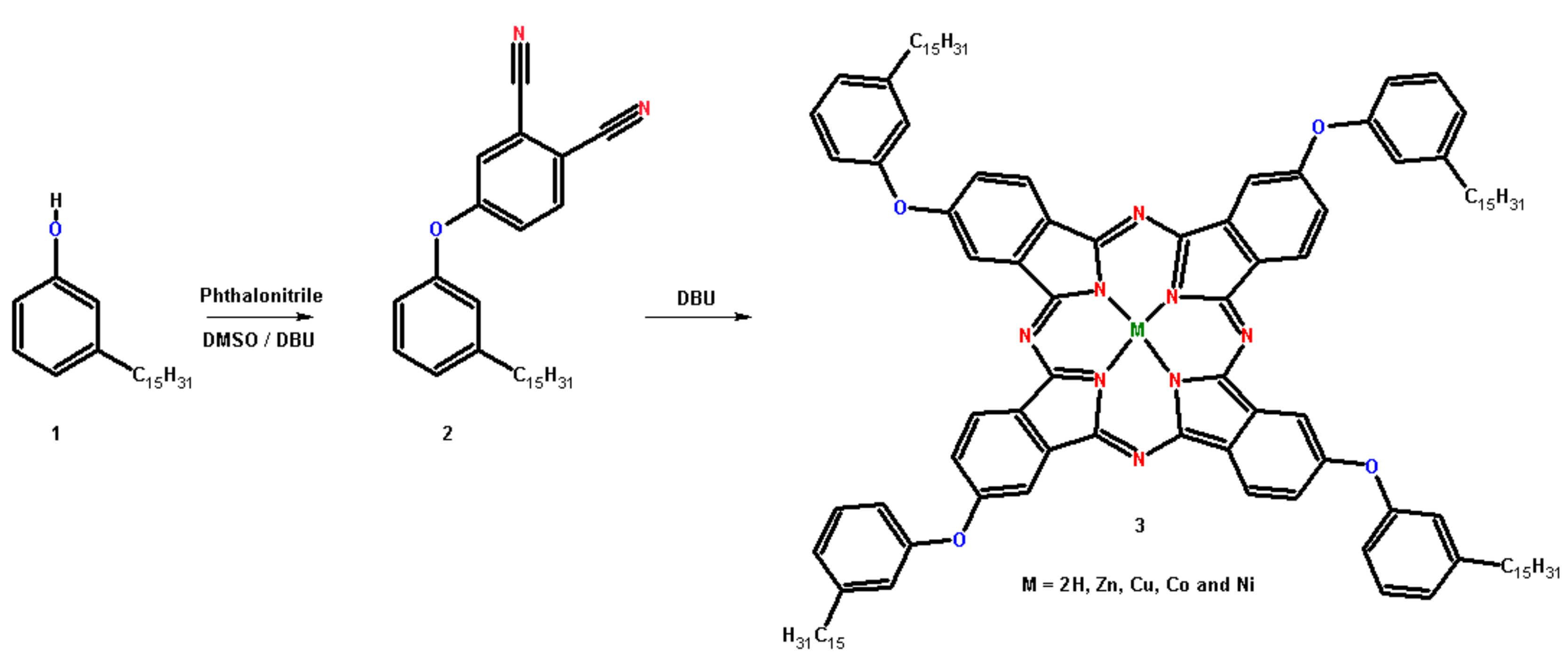
3.1. Synthesis and Metallation of Phthalocyanines from CNSL
3.2. Synthesis of Magnetite
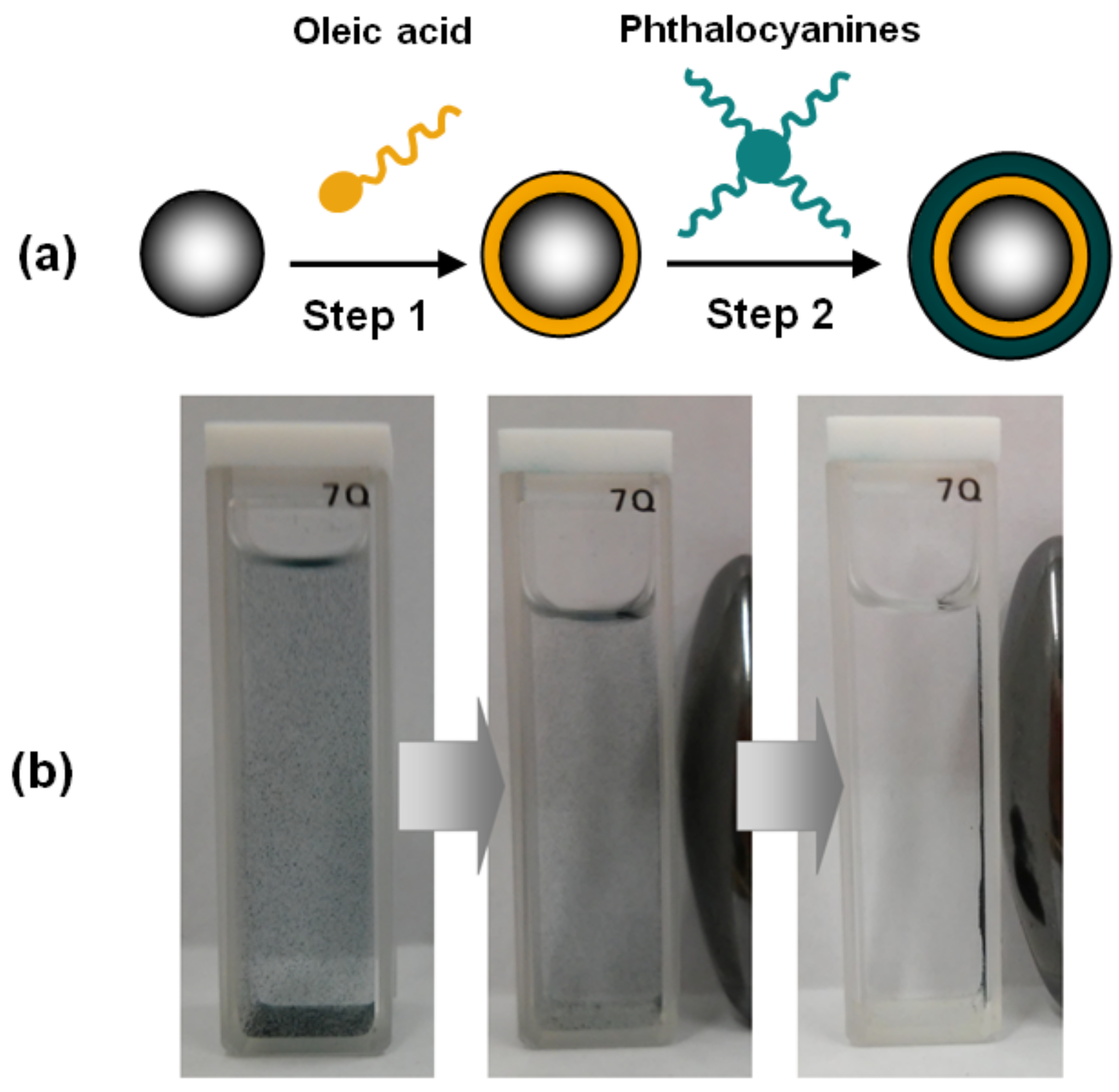
3.3. Preparation of Magnetic Nanomaterials
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, A.K.; Srivastava, O.N.; Singh, K. Shape and size-dependent magnetic properties of Fe3O4 nanoparticles synthesized using piperidine. Nanoscale Res. Lett. 2017, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Zinadini, S.; Alizadeh, R.; Heydari, R.; Beygzadeh, M.; Ghouzivand, S. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263, 101–112. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Rasika Dias, H.V.; Méndez, U.O.; La Fuente, I.G.; Peña, Y.; Dimas, A.V. Iron-based nanomaterials in the catalysis. In Advanced Catalytic Materials—Photocatalysis and Other Current Trends, 1st ed.; Luis, N., Ed.; IntechOpen: London, UK, 2016; Volume 2, pp. 35–68. [Google Scholar]
- Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe3O4 nanoparticles for complex targeted delivery and boron neutron capture therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Joshi, P.; Zhou, Y.; Ding, R.; Zhang, P. Quantitative SERS-based DNA detection assisted by magnetic microspheres. Chem. Commun. 2015, 51, 15284–15286. [Google Scholar] [CrossRef] [PubMed]
- Nyokong, T.; Antunes, E. Influence of nanoparticle materials on the photophysical behavior of phthalocyanines. Coord. Chem. Rev. 2013, 257, 2401–2418. [Google Scholar] [CrossRef]
- Er, O.; Colak, S.G.; Ocakoglu, K.; Ince, M.; Bresolí-Obach, R.; Mora, M.; Sagristá, M.L.; Yurt, F.; Nonell, S. Selective photokilling of human pancreatic cancer cells using cetuximab-targeted mesoporous silica nanoparticles for delivery of zinc phthalocyanine. Molecules 2018, 23, 2749. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wei, W.; Hong, M.; Jianfeng, C.; Guangwen, C.; Haikui, Z. Microwave assisted synthesis of ZnPc-COOH and SiO2/ZnPc-COOH nanopaticles: Singlet oxygen production and photocatalytic property. Colloids Surf. A Physicochem. Eng. Aspects 2014, 443, 52–59. [Google Scholar] [CrossRef]
- Modisha, P.; Nyokong, T. Fabrication of phthalocyanine-magnetic nanoparticles hybrid nanofibers for degradation of Orange-G. J. Mol. Catal. A Chem. 2014, 381, 132–137. [Google Scholar] [CrossRef]
- Gomis, L.M.; Lázaro, F.F.; Santos, A.S. Advances in phthalocyanine-sensitized solar cells (PcSSCs). J. Mater. Chem. A 2014, 2, 15672–15682. [Google Scholar] [CrossRef]
- Basova, T.V.; Parkhomenko, R.G.; Igumenov, I.K.; Hassan, A.; Durmus, M.; Gürek, A.G.; Ahsen, V. Composites of liquid crystalline nickel phthalocyanine with gold nanoparticles: Liquid crystalline behaviour and optical properties. Dyes Pigments 2014, 111, 58–63. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Hua, X.C.; Zhai, T.S.; Li, Y.H.; Lu, X.; Duhm, S.; Fung, M.K. Doped copper phthalocyanine via an aqueous solution process for high-performance organic light-emitting diodes. Org. Electron. 2019, 68, 236–241. [Google Scholar] [CrossRef]
- Özcesmeci, I.; Gelir, A.; Gül, A. Synthesis and photophysical properties of indium (III) phthalocyanine derivatives. J. Lumin. 2014, 147, 141–146. [Google Scholar] [CrossRef]
- Awaji, A.I.; Köksoy, B.; Durmuş, M.; Aljuhani, A.; Alraqa, S.Y. Novel hexadeca-substituted metal free and zinc(II) phthalocyanines; Design, synthesis and photophysicochemical properties. Molecules 2019, 24, 77. [Google Scholar] [CrossRef] [PubMed]
- Attanasi, O.A.; Ciccarella, G.; Filippone, P.; Mele, G.; Spadavecchia, J.; Vasapollo, G. Novel phthalocyanines containing cardanol derivatives. J. Porphy. Phthalocyanines 2003, 7, 52–57. [Google Scholar] [CrossRef]
- Slota, R.; Dyrda, G.; Hofer, M.; Mele, G.; Bloise, E.; Del Sole, R. Novel lipophilic lanthanide bis-phthalocyanines functionalized by pentadecylphenoxy groups: Synthesis, characterization and UV-photostability. Molecules 2012, 17, 10738–10753. [Google Scholar] [CrossRef] [PubMed]
- Costa Junior, A.E.; Mota, J.P.F.; Pontes, S.M.A.; Maia, F.J.N.; Clemente, C.S.; Fechine, P.B.A.; Bohn, F.; Sales, A.J.M.; Sombra, A.S.B.; Carbone, L.; et al. A self-assembly of graphene oxide@Fe3O4/metallo-phthalocyanine nanohybrid materials: Synthesis, characterization, dielectric and thermal properties. J. Mater. Sci. 2017, 52, 9546–9557. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Marcelo, A.M.P.; da Silva, K.T.; da Silva, F.L.F.; Mota, J.P.F.; do Nascimento, J.P.C.; Sombra, A.S.B.; da Silva Clemente, C.; Mele, G.; Carbone, L.; et al. New ZnO@Cardanol porphyrin composite nanomaterials with enhanced photocatalytic aapability under solar light irradiation. Materials 2017, 10, 1114. [Google Scholar] [CrossRef]
- Lima, N.M.A.; Avila, H.J.C.; Marchiori, C.F.N.; Sampaio, S.G.; Mota, J.P.F.; Ribeiro, V.G.P.; Clemente, C.S.; Mele, G.; Cremona, M.; Mazzetto, S.E. Light-emitting porphyrin derivative obtained from a subproduct of the cashew nut shell liquid: A promising material for OLED applications. Materials 2019, 12, 1063. [Google Scholar]
- Mota, J.P.F.; Ribeiro, V.G.P.; da Silva, F.L.F.; Costa Junior, A.E.; Oliveira, D.R.; Kotzebue, L.R.V.; Mele, G.; Lomonaco, D.; Mazzetto, S.E. Developing eco-friendly methods for purification of compounds derived from hydrogenated cardanol. Sep. Sci. Technol. 2016, 51, 2473–2483. [Google Scholar] [CrossRef]
- Lomonaco, D.; Mele, G.; Mazzetto, S.E. Cashew Nut Shell Liquid (CNSL): Form an agro-industrial waste to a sustainable alternative to petrochemical resources. In Cashew Nut Shell Liquid, 1st ed.; Anilkumar, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 19–38. [Google Scholar]
- Mota, J.P.F.; Costa Junior, A.E.; Ribeiro, V.G.P.; Sampaio, S.G.; Lima, N.M.A.; Clemente, C.S.; Mele, G.; Lomonaco, D.; Mazzetto, S.E. Synthesis, characterization and dielectric properties of new 5-(4-hydroxyphenyl)-10,15,20-tri-4-[2-(3-pentadecylphenoxy)ethoxy]phenyl porphyrin and their Ni, Co and Cu complexes. J. Braz. Chem. Soc. 2016, 28, 1063–1073. [Google Scholar] [CrossRef]
- Mele, G.; Lomonaco, D.; Mazzetto, S.E. Cardanol-based heterocycles: Synthesis and applications. In Cashew Nut Shell Liquid, 1st ed.; Anilkumar, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 39–56. [Google Scholar]
- Yan, L.; Pu, Z.; Xu, M.; Wei, R.; Liu, X. Fabrication and Electromagnetic Properties of Conjugated NH2-CuPc@Fe3O4. J. Electron. Mater. 2017, 46, 5608–5618. [Google Scholar] [CrossRef]
- Liu, S.; Liu, C.; Liu, C.; Tu, L.; You, Y.; Wei, R.; Liu, X. Polyarylene ether nitrile and barium titanate nanocomposite plasticized by carboxylated zinc phthalocyanine buffer. Polymers 2019, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Matlou, G.G.; Oluwole, D.O.; Prinsloo, E.; Nyokong, T. Photodynamic therapy activity of zinc phthalocyanine linked to folic acidand magnetic nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 186, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zheng, P.; Tong, L.; Ren, D.; Xu, M.; Tang, X.; Liu, X. Design and properties of Poly(arylene ether nitriles) composites via incorporation of Poly(arylene ether nitriles) grafted Fe3O4/ Fe-phthalocyanine hybrid submicron-spheres. Compos. Part. B 2019, 176, 107202–107210. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, R.; Zhan, Y.; Lei, Y.; Zhong, J.; Liu, X. One-step synthesis of Fe-phthalocyanine/Fe3O4 hybrid microspheres. Mater. Lett. 2011, 65, 264–267. [Google Scholar] [CrossRef]
- Karimi, A.R.; Azadikhah, F.; Rahimi, L.; Ghadimi, S. Fabrication of new Fe-phthalocyanine oligomer–magnetite hybrid magnetic nano particles and their effects on the LCST behavior of thermo-sensitive poly(N-isopropylacrylamide-co-acrylic acid) magnetic nanocomposites. Colloids Surf. A Physicochem. Eng. Aspects 2015, 484, 304–312. [Google Scholar] [CrossRef]
- Li, K.; Ren, D.; Tang, X.; Xu, M.; Liu, X. Micro/mesoporous Fe3O4/Fe-phthalocyanine microspheres and effects of their surface morphology on the crystallization and properties of poly(arylene ether nitrile) composites. Materials 2018, 11, 1356. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef]
- Bayrak, R.; Dumludag, F.; Akçay, H.T.; Degirmencioglu, I. Synthesis, characterization and electrical properties of peripherally tetra-aldazine substituted novel metal free phthalocyanine and its zinc (II) and nickel (II) complexes. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2013, 105, 550–556. [Google Scholar] [CrossRef]
- Modisha, P.; Nyokong, T.; Antunes, E. Photodegradation of Orange-G using zinc octacarboxyphthalocyanine supported on Fe3O4 nanoparticles. J. Mol. Catal. A Chem. 2013, 380, 131–138. [Google Scholar] [CrossRef]
- Barreto, A.C.H.; Maia, F.J.N.; Santiago, V.R.; Ribeiro, V.G.P.; Denardin, J.C.; Mele, G.; Carbone, L.; Lomonaco, D.; Mazzetto, S.E.; Fechine, P.B.A. Novel ferrofluids coated with a renewable material obtained from cashew nut shell liquid. MicrofluidNanofluid 2012, 12, 677–686. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Barreto, A.C.H.; Denardin, J.C.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Sousa, E.M.B.; Fechine, P.B.A. Magnetic nanoparticles coated with anacardic acid derived from cashew nut shell liquid. J. Mater. Sci. 2013, 48, 7875–7882. [Google Scholar] [CrossRef]
- Souza, N.D.G.; Freire, R.M.; Cunha, A.P.; da Silva, M.A.S.; Mazzetto, S.E.; Sombra, A.S.B.; Denardin, J.C.; Ricardo, N.M.P.S.; Fechine, P.B.A. New magnetic nanobiocomposite based in galactomannan/glycerol and superparamagnetic nanoparticles. Mater. Chem. Phys. 2015, 156, 113–120. [Google Scholar] [CrossRef]
- Li, J.; Wei, J.; Pu, Z.; Xu, M.; Jia, K.; Liu, X. Influence of Fe3O4/Fe-phthalocyanine decorated graphene oxide on the microwave absorbing performance. J. Magn. Magn. Mater. 2016, 399, 81–87. [Google Scholar] [CrossRef]
- Sevim, A.M.; Yenilmez, H.Y.; Bayir, Z.A. Synthesis and photophysical properties of novel (trifluoromethyl)phenylethynyl-substituted metallophthalocyanines. Polyhedron 2013, 62, 120–125. [Google Scholar] [CrossRef]
- Kaya, E.C.; Durmus, M.; Yanmaz, E.; Kantekin, H. Synthesis and spectral and thermal characterization of new metal-free and metallophthalocyanines: Investigation of their photophysical, photochemical, and thin film properties. Turk. J. Chem. 2014, 38, 1118–1134. [Google Scholar] [CrossRef]
- Mele, G.; Vasapollo, G. Fine chemicals and new hybrid materials from cardanol. Mini Rev. Org. Chem. 2008, 5, 243–253. [Google Scholar] [CrossRef]
- Dilber, G.; Durmus, M.; Kantekin, H.; Çakir, V. Synthesis and characterization of a new soluble metal-free and metallophthalocyanines bearing biphenyl-4-yl methoxy groups. J. Org. Chem. 2011, 696, 2805–2814. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
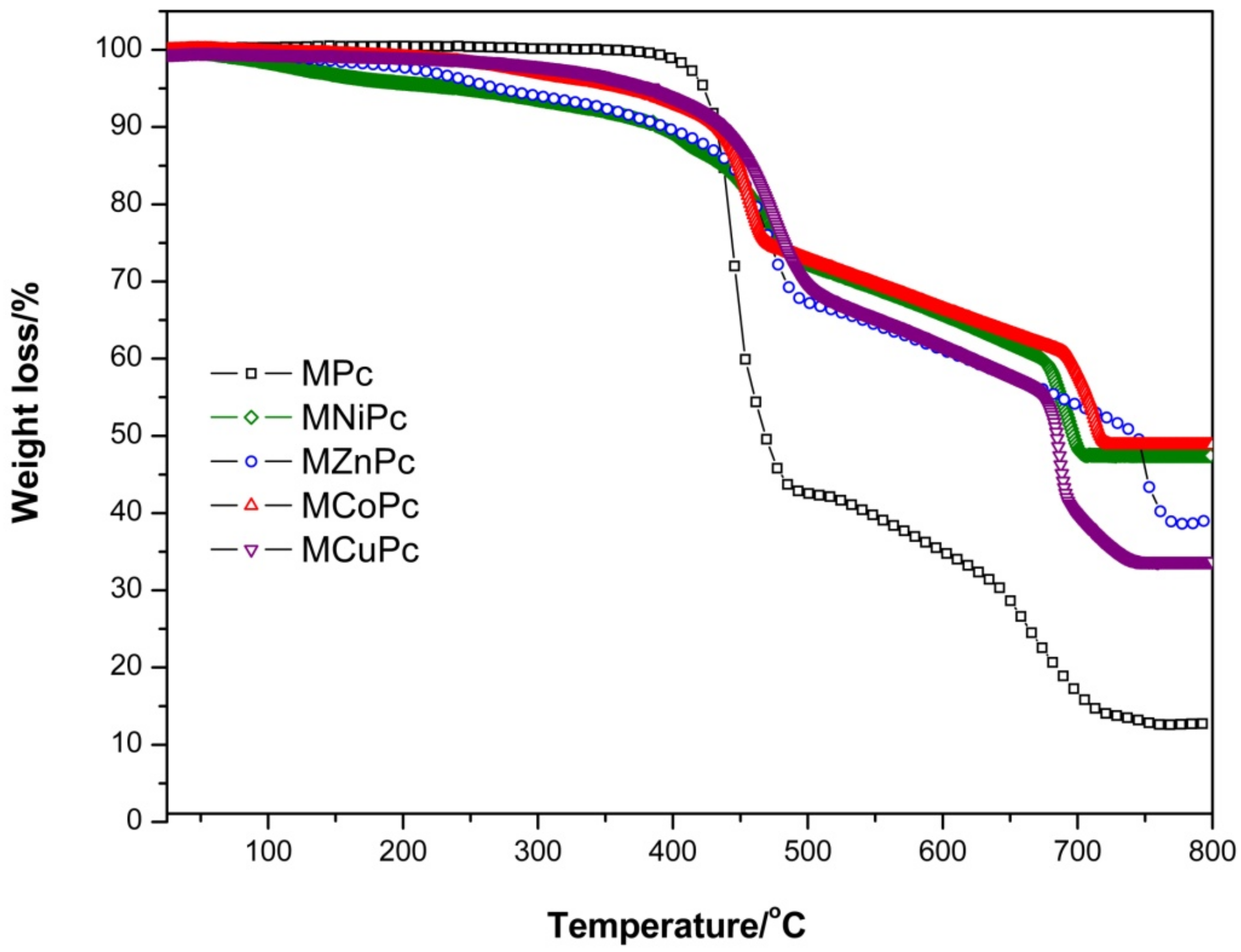

| Nanomaterials | First Stage | Second Stage | Residue/% | ||
|---|---|---|---|---|---|
| Range/°C | Mass Loss/% | Range/°C | Mass Loss/% | ||
| Fe3O4@OA/Pc | 386–603 | 65.00 | 615–800 | 22.22 | 12.32 |
| Fe3O4@OA/CoPc | 292–673 | 35.39 | 675–641 | 12.88 | 48.71 |
| Fe3O4@OA/CuPc | 285–690 | 42.10 | 692–725 | 22.18 | 34.32 |
| Fe3O4@OA/NiPc | 255–673 | 34.71 | 675–707 | 12.53 | 47.95 |
| Fe3O4@OA/ZnPc | 167–740 | 47.74 | 741–784 | 12.07 | 38.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, V.G.P.; Mota, J.P.F.; Costa Júnior, A.E.; Lima, N.M.A.; Fechine, P.B.A.; Denardin, J.C.; Carbone, L.; Bloise, E.; Mele, G.; Mazzetto, S.E. Nanomaterials Based on Fe3O4 and Phthalocyanines Derived from Cashew Nut Shell Liquid. Molecules 2019, 24, 3284. https://doi.org/10.3390/molecules24183284
Ribeiro VGP, Mota JPF, Costa Júnior AE, Lima NMA, Fechine PBA, Denardin JC, Carbone L, Bloise E, Mele G, Mazzetto SE. Nanomaterials Based on Fe3O4 and Phthalocyanines Derived from Cashew Nut Shell Liquid. Molecules. 2019; 24(18):3284. https://doi.org/10.3390/molecules24183284
Chicago/Turabian StyleRibeiro, Viviane G. P., João P. F. Mota, Antônio E. Costa Júnior, Nayane M. A. Lima, Pierre B. A. Fechine, Juliano C. Denardin, Luigi Carbone, Ermelinda Bloise, Giuseppe Mele, and Selma E. Mazzetto. 2019. "Nanomaterials Based on Fe3O4 and Phthalocyanines Derived from Cashew Nut Shell Liquid" Molecules 24, no. 18: 3284. https://doi.org/10.3390/molecules24183284
APA StyleRibeiro, V. G. P., Mota, J. P. F., Costa Júnior, A. E., Lima, N. M. A., Fechine, P. B. A., Denardin, J. C., Carbone, L., Bloise, E., Mele, G., & Mazzetto, S. E. (2019). Nanomaterials Based on Fe3O4 and Phthalocyanines Derived from Cashew Nut Shell Liquid. Molecules, 24(18), 3284. https://doi.org/10.3390/molecules24183284







