Combined Analysis of Primary Metabolites and Phenolic Compounds to Authenticate Commercial Monovarietal Peach Purees and Pear Juices
Abstract
:1. Introduction
2. Results and Discussion
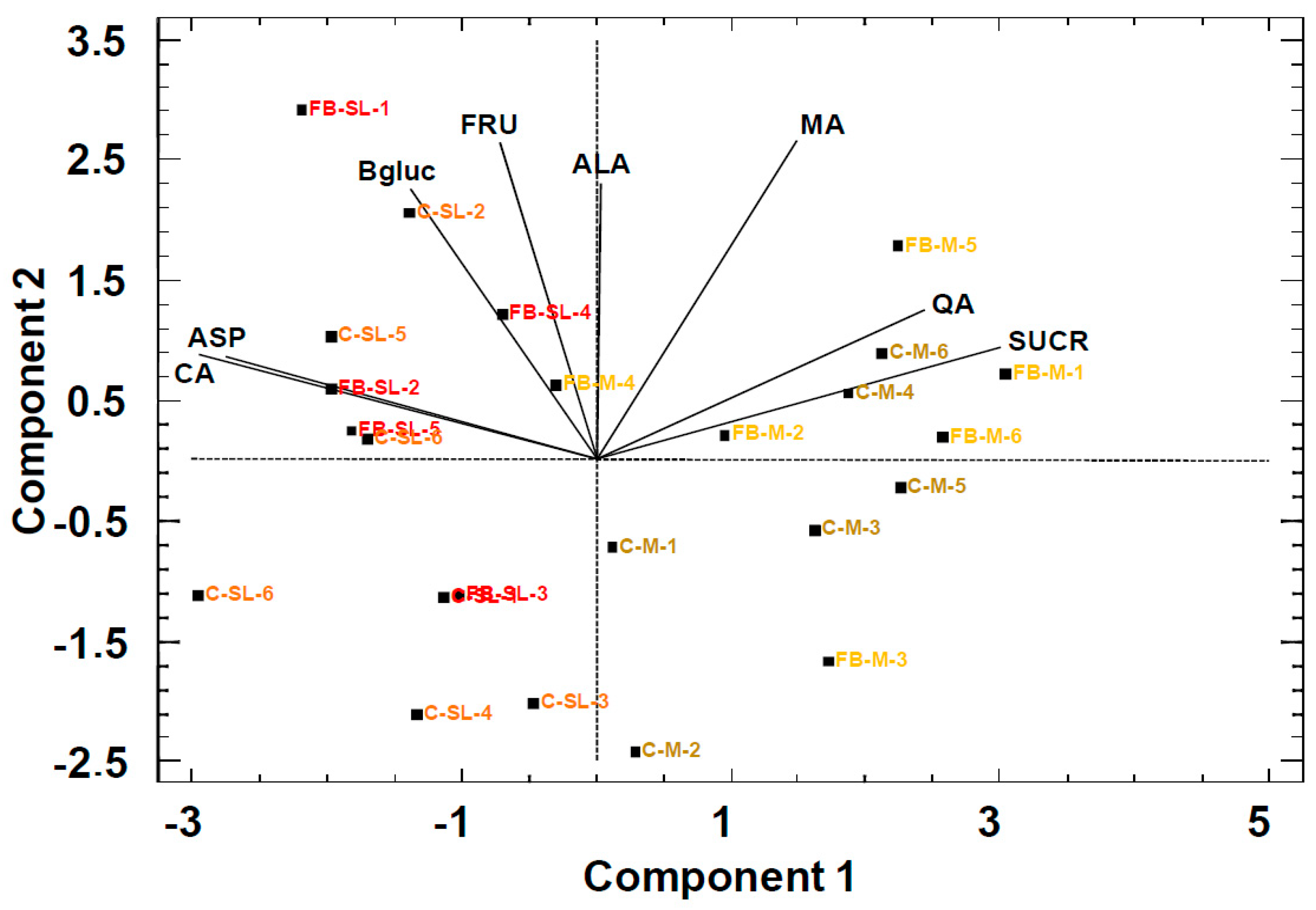
2.1. 1H NMR and Chemometric Analysis of Primary Metabolites
2.2. Chemometric Analysis of Phenolic Compounds Determined by Ultra Performance Liquid Chromatography coupled to Photodiode Array, Fluorescence and Tandem Mass Spectrometry UPLC-PDA-FLR-MS/MS
2.3. Combination of 1H NMR and UPLC-PDA-FLR-MS/MS Chemometric Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Sample Preparation
3.4. H NMR Analysis
3.5. UPLC Analysis. UPLC-PDA-FLR Parameters
3.6. UPLC-MS/MS Parameters
3.7. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- European Fruit Juice Association (AIJN). Available online: https://aijn.eu/en/publications/market-reports-1/publication-2 (accessed on 8 December 2017).
- Johnston, M.R.; Kauffman, F. Orange juice adulteration detection and actions of the FDA. J. Food Qual. 1985, 8, 81–89. [Google Scholar] [CrossRef]
- Marieschi, M.; Torelli, A.; Beghe, D.; Bruni, R. Authentication of Punica granatum L.: Development of SCAR markers for the detection of 10 fruits potentially used in economically motivated adulteration. Food Chem. 2016, 202, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Cuny, M.; Vigneau, E.; Le Gall, G.; Colquhoun, I.; Lees, M.; Rutledge, D. Fruit juice authentication by 1H NMR spectroscopy in combination with different chemometrics tools. Anal. Bioanal. Chem. 2008, 390, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Antolovich, M. Methods for assessing the authenticity of orange juice. A review. Analyst 1995, 120, 1–28. [Google Scholar] [CrossRef]
- Vaclavik, L.; Schreiber, A.; Lacina, O.; Cajka, T.; Hajslova, J. Liquid chromatography–mass spectrometry-based metabolomics for authenticity assessment of fruit juices. Metabolomics 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Mondello, L.; Tranchida, P.Q.; Dugo, P.; Dugo, G. Comprehensive two--dimensional gas chromatography--mass spectrometry: A review. Mass Spectrom. Rev. 2008, 27, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Downey, G. Review: Authentication of food and food ingredients by near infrared spectroscopy. J. Near Infrared Spectrosc. 1997, 4, 47–61. [Google Scholar] [CrossRef]
- Belton, P.S.; Colquhoun, I.J.; Kemsley, E.K.; Delgadillo, I.; Roma, P.; Dennis, M.J.; Sharman, M.; Holmes, E.; Nicholson, J.K.; Spraul, M. Application of chemometrics to the 1H NMR spectra of apple juices: Discrimination between apple varieties. Food Chem. 1998, 61, 207–213. [Google Scholar] [CrossRef]
- Ogrinc, N.; Košir, I.J.; Spangenberg, J.E.; Kidrič, J. The application of NMR and MS methods for detection of adulteration of wine, fruit juices, and olive oil. A review. Anal. Bioanal. Chem. 2003, 376, 424–430. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R.; Cisneros-Zevallos, L. Selecting new peach and plum genotypes rich in phenolic compounds and enhanced functional properties. Food Chem. 2006, 96, 273–280. [Google Scholar] [CrossRef]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann. Appl. Biol. 2008, 153, 11–23. [Google Scholar] [CrossRef]
- Capitani, D.; Sobolev, A.P.; Tomassini, A.; Sciubba, F.; De Salvador, F.R.; Mannina, L.; Delfini, M. Peach fruit: Metabolic comparative analysis of two varieties with different resistances to insect attacks by NMR spectroscopy. J. Agric. Food Chem. 2012, 61, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Tanrıöven, D.; Ekşi, A. Phenolic compounds in pear juice from different cultivars. Food Chem. 2005, 93, 89–93. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef] [PubMed]
- Kevers, C.; Pincemail, J. l.; Tabart, J.; Defraigne, J.-O.; Dommes, J. Influence of cultivar, harvest time, storage conditions, and peeling on the antioxidant capacity and phenolic and ascorbic acid contents of apples and pears. J. Agric. Food Chem. 2011, 59, 6165–6171. [Google Scholar] [CrossRef] [PubMed]
- Spanos, G.A.; Wrolstad, R.E. Phenolics of apple, pear, and white grape juices and their changes with processing and storage. A review. J. Agric. Food Chem. 1992, 40, 1478–1487. [Google Scholar] [CrossRef]
- Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Liquid chromatography/mass spectrometry investigation of the impact of thermal processing and storage on peach procyanidins. J. Agric. Food Chem. 2004, 52, 2366–2371. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Processing-induced changes in total phenolics and procyanidins in clingstone peaches. J. Sci. Food Agric. 2003, 83, 56–63. [Google Scholar] [CrossRef]
- Basak, S.; Ramaswamy, H. Effect of high pressure processing on the texture of selected fruits and vegetables. J. Texture Stud. 1998, 29, 587–601. [Google Scholar] [CrossRef]
- Hui, Y.H. Handbook of Fruits and Fruit Processing, 1st ed.; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Abad-Garcia, B.; Garmon-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. Practical guidelines for characterization of O-diglycosyl flavonoid isomers by triple quadrupole MS and their applications for identification of some fruit juices flavonoids. J. Mass Spectrom. 2009, 44, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Scordino, M.; Sabatino, L.; Muratore, A.; Belligno, A.; Gagliano, G. Phenolic characterization of sicilian yellow flesh peach (prunus persicaL.) cultivars at different ripening stages. J. Food Qual. 2012, 35, 255–262. [Google Scholar] [CrossRef]
- Ferreira, D.; Guyot, S.; Marnet, N.; Delgadillo, I.; Renard, C.M.; Coimbra, M.A. Composition of phenolic compounds in a Portuguese pear (Pyrus communis L. var. S. Bartolomeu) and changes after sun-drying. J. Agric. Food Chem. 2002, 50, 4537–4544. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Impact of juice processing on blueberry anthocyanins and polyphenolics: Comparison of two pretreatments. J. Food Sci. 2002, 67, 1660–1667. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Compound 2 | Assignment | 1H (ppm Hz) |
|---|---|---|
| Alanine (ALA) | β-CH3 | 1.49 |
| Quinic acid (QA) | CH2-1,1′; CH2-5,5′ | 1.7 |
| Citric acid (CA) | α,γ-CH | 2.3 |
| Asparagine (ASP) | β, β′-CH2 | 2.7 |
| β-Glucose (βGLC) | CH-2 | 2.95 |
| β-D-Fructopyranose (FRU) | CH2-6,6′ | 3.85 |
| Malic acid (MA) | β, β′-CH2 | 4.1 |
| α-Glucose (αGLC) | CH-1 | 4.95 |
| Sucrose (SUCR) | GLC CH-1 | 5.15 |
| Maleic acid (IS) | 2CH | 5.9 |
| Retention Time (min) | Compound1 | UV Max (nm) | [M − H]−/[M + H]+ (m/z) | Relative Abundances of Main Ions from MS/MS of [M − H]−/[M + H]+ | CE (eV)4 |
|---|---|---|---|---|---|
| 2.53 | Hydroquinone β-d-glucopyranoside or Arbutin2 (Arb) | 283 | 271/273 | 108(100), 95 (18)/− | 20 |
| 5.85 | Protocatechuic aldehyde monoglucuronide3 (ProCat-glu) | 268 | 313/315 | 175/− | 20 |
| 6.45 | 3-Caffeoylquinic acid3 (3-Caf-qui) | 240, 326 | 353/355 | 191 (100), 179 (48)/−/ | 20 |
| 7.02 | Coumaroyl-hexose3 (Cou-hex) | 316 | 325/327 | 163/- | 20 |
| 7.68 | Procyanidin-B12 (Pro-B1) | 278 | 289/291 | 407(100), 289(63), 425 (34),/291(100), 409(88), 427(64), 247(64), 301(64) | 20 |
| 7.96 | 5-Caffeoylquinic acid2 (5-Caf-qui) | 240, 326 | 353/356 | 191 (100), 179 (94)/163(100), 145(62), 135(20), 117(20) | 20 |
| 8.22 | (+)-Catechin2 (Cat) | 278 | 289/291 | 139(100), 123(47), 147(22)/203(100), 109(94), 125(84) | 20 |
| 8.43 | 4-Caffeoylquinic acid3 (4-Caf-qui) | 240, 326 | 353/355 | 173 (100), 179(67),191(32) /− | 20 |
| 9.23 | Procyanidin-B22 (Pro-B2) | 278 | 577/579 | 291(100), 409(88), 427(64), 247(64), 301(64) /407(100), 289(63), 425 (34) | 20 |
| 9.47 | 5-p-Coumaroylquinic acid3 (5-p-Cou-qui) | 235,312 | 337/339 | 191(100), 163(22)/− | 20 |
| 9.88 | (-)-Epicatechin2 (Epi) | 278 | 289/291 | 139(100), 123(47), 147(22)/203(100), 109(94), 125(84) | 20 |
| 12.49 | Quercetin-3-O-rutinoside2 (Q-rut) | 255, 353 | 609/611 | 300(100)/303(100) | 30 |
| 12.59 | Quercetin-3-O-galactoside2 (Q-gal) | 255, 353 | 463/465 | 300(100), 271(27), 255(19)/303(100), 153(26) | 30 |
| 12.82 | Quercetin-3-O-glucoside2 (Q-glu) | 255, 353 | 463/465 | 300(100), 271(27), 255(19)/303(100), 153(26) | 30 |
| 13.64 | Kaempferol 3-O-rutinoside2 (K-rut) | 265, 347 | 593/595 | 287/− | 30 |
| 13.8 | Isorhamnetin-3-O-robinioside3 (Iso-rob) | 254, 352 | 623/625 | 317/− | 30 |
| 13.98 | Isorhamnetin-3-O-rutinoside2 (Iso-rut) | 254, 352 | 623/625 | 317/− | 30 |
| 14.12 | Isorhamnetin-3-O-galactoside3 (Iso-gal) | 254, 352 | 477/479 | 317/− | 30 |
| 14.38 | Isorhamnetin-3-O-glucoside3 (Iso-glu) | 254, 352 | 477/479 | 317/− | 30 |
| 15.05 | Isorhamnetin 3-O-malonygalactoside3 (Iso-malgal) | 254, 352 | 563/565 | 317/− | 30 |
| 15.36 | Isorhamnetin 3-O-6″-malonygalactoside3 (Iso-6″-malgal) | 254, 352 | 563/565 | 317/− | 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delpino-Rius, A.; Eras, J.; Gatius, F.; Balcells, M.; Canela-Garayoa, R. Combined Analysis of Primary Metabolites and Phenolic Compounds to Authenticate Commercial Monovarietal Peach Purees and Pear Juices. Molecules 2019, 24, 3289. https://doi.org/10.3390/molecules24183289
Delpino-Rius A, Eras J, Gatius F, Balcells M, Canela-Garayoa R. Combined Analysis of Primary Metabolites and Phenolic Compounds to Authenticate Commercial Monovarietal Peach Purees and Pear Juices. Molecules. 2019; 24(18):3289. https://doi.org/10.3390/molecules24183289
Chicago/Turabian StyleDelpino-Rius, Antoni, Jordi Eras, Ferran Gatius, Mercè Balcells, and Ramon Canela-Garayoa. 2019. "Combined Analysis of Primary Metabolites and Phenolic Compounds to Authenticate Commercial Monovarietal Peach Purees and Pear Juices" Molecules 24, no. 18: 3289. https://doi.org/10.3390/molecules24183289
APA StyleDelpino-Rius, A., Eras, J., Gatius, F., Balcells, M., & Canela-Garayoa, R. (2019). Combined Analysis of Primary Metabolites and Phenolic Compounds to Authenticate Commercial Monovarietal Peach Purees and Pear Juices. Molecules, 24(18), 3289. https://doi.org/10.3390/molecules24183289






