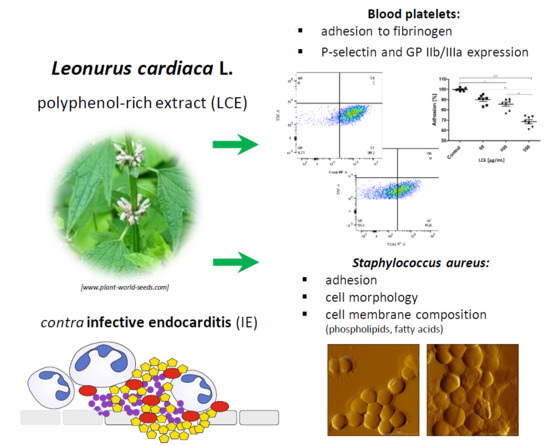
Molecular Mechanisms of Leonurus Cardiaca L. Extract Activity in Prevention of Staphylococcal Endocarditis—Study on in Vitro and ex Vivo Models
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Bacterial Strain
3.2. Platelets
3.3. Preparation of L. cardiaca L. Extract (LCE) Solutions
3.4. LCE Impact on S. Aureus Adhesive Properties Tested Using Atomic Force Microscopy (AFM)
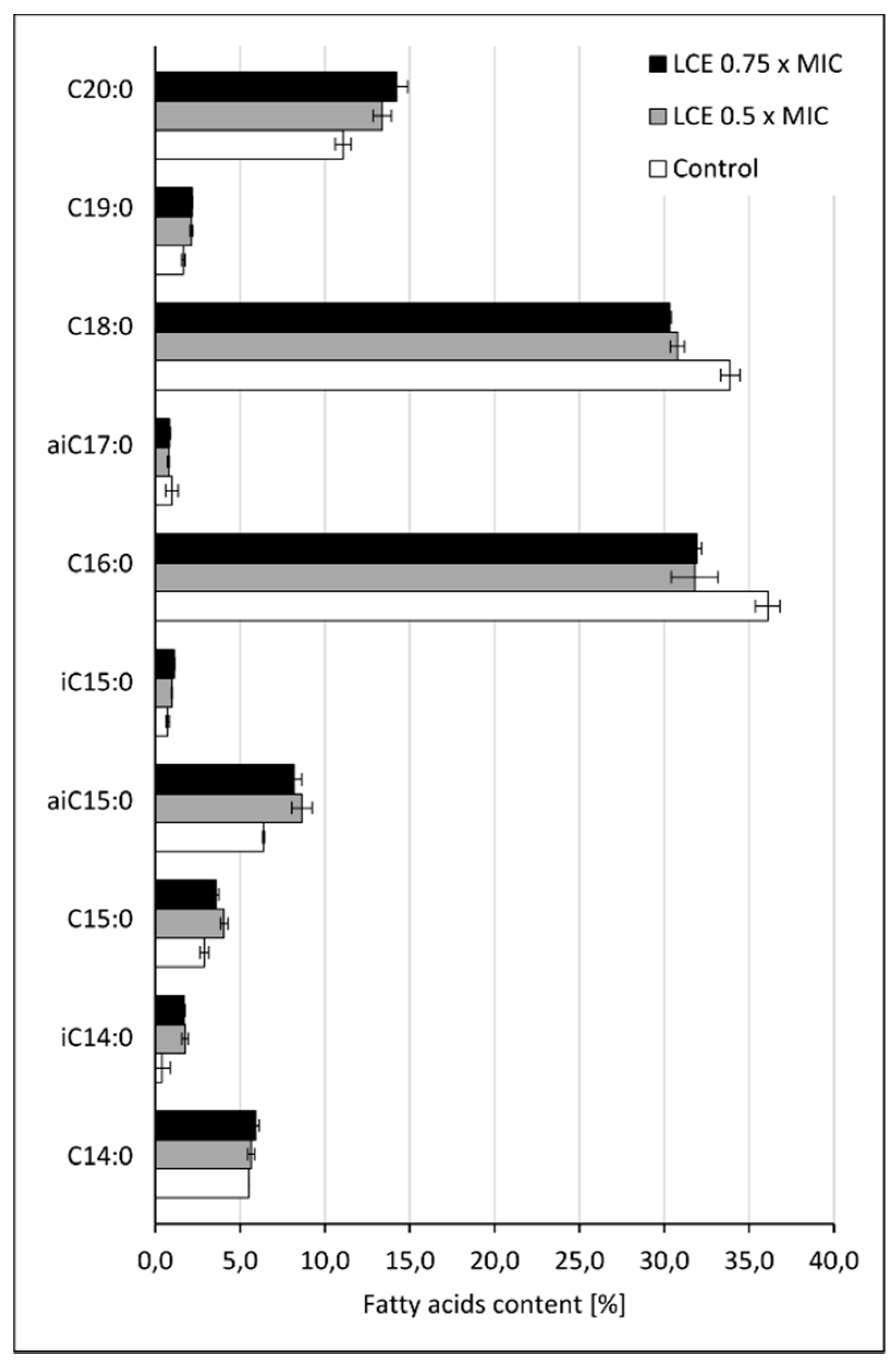
3.5. The Effect of LCE on Staphylococcal Cell Membrane Lipid Profile
3.5.1. Chemicals
3.5.2. Phosholipid Extraction and HPLC Analysis
3.5.3. Fatty Acids Extraction and GC-MS Analysis
3.6. LCE Influence on Blood Platelet Adhesion to Fibrinogen
3.7. Flow Cytometric Analysis of Blood Platelets Activation after Exposure to LCE
3.8. Statistics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Keynan, Y.; Rubinstein, E. Pathophysiology of infective endocarditis. Curr. Infect. Dis. Rep. 2013, 15, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, P.; Nahrendorf, M.; Figueiredo, J.-L.; Panizzi, J.; Marinelli, B.; Iwamoto, Y.; Keliher, E.; Maddur, A.A.; Waterman, P.; Kroh, H.K.; et al. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat. Med. 2011, 17, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-K. Infective endocarditis involving an apparently structurally normal valve: New epidemiological trend? Korean J. Intern. Med. 2015, 30, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Claes, J.; Liesenborghs, L.; Peetermans, M.; Veloso, T.; Missiakas, D.; Schneewind, O.; Mancini, S.; Entenza, J.M.; Hoylaerts, M.F.; Heying, R.; et al. Clumping factor A, von Willebrand factor-binding protein and von Willebrand factor anchor Staphylococcus aureus to the vessel wall. J. Thromb. Haemost. 2017, 15, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Intern. J. Antim. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Leid, J.G. Bacterial biofilms resist key host defenses. Microbe 2009, 4, 66–70. [Google Scholar]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivas Morales, C.; Castillo, S.; Leos-Rivas, C.; García-Becerra, L.; Martínez, D.M.O. Antibacterial and antibiofilm activity of methanolic plant extracts against nosocomial microorganisms. Evi.-Based Complem. Altern. Med. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Kaiser, S.J.; Mutters, N.T.; Blessing, B.; Günther, F. Natural isothiocyanates express antimicrobial activity against developing and mature biofilms of Pseudomonas aeruginosa. Fitoterapia 2017, 119, 57–63. [Google Scholar] [CrossRef]
- Souza, L.B.F.C.; Silva-Rocha, W.P.; Ferreira, M.R.A.; Soares, L.A.L.; Svidzinski, T.I.E.; Milan, E.P.; Pires, R.H.; Almeida, A.M.F.; Mendes-Giannini, M.J.S.; Chaves, G.M. Influence of Eugenia uniflora extract on adhesion to human buccal epithelial cells, biofilm formation, and cell surface hydrophobicity of Candida spp. from the oral cavity of kidney transplant recipients. Molecules 2018, 23, 2418. [Google Scholar] [CrossRef] [PubMed]
- Micota, B.; Sadowska, B.; Podsędek, A.; Paszkiewicz, M.; Sosnowska, D.; Różalska, B. Is it true that plant-derived polyphenols are always beneficial for the human? In vitro study on Leonurus cardiaca extract properties in the context of the pathogenesis of Staphylococcus aureus infections. J. Med. Microb. 2016, 65, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, B.; Micota, B.; Różalski, M.; Redzynia, M.; Różalski, M. The immunomodulatory potential of Leonurus cardiaca extract in relation to endothelial cells and platelets. Innate Immun. 2017, 23, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Micota, B.; Sadowska, B.; Podsędek, A.; Redzynia, M.; Różalska, B. Leonurus cardiaca L. herb—A derived extract and an ursolic acid as a factor affecting the adhesion capacity of Staphylococcus aureus in the context of infective endocarditis. Acta Biochim. Pol. 2014, 61, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M.; Jakstas, V.; Majiene, D.; Baniene, R.; Kuršvietiene, L.; Masteikova, R.; Savickas, A.; Toleikis, A.; Trumbeckaite, S. The effect of Leonurus cardiaca herb extract and some of its flavonoids on mitochondrial oxidative phosphorylation in the heart. Planta Med. 2014, 80, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Wojtyniak, K.; Szymański, M.; Matławska, I. Leonurus cardiaca L. (Motherwort): A review of its phytochemistry and pharmacology. Phytother. Res. 2013, 27, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Formosa-Daguea, C.; Duval, R.E.; Dague, E. Cell biology of microbes and pharmacology of antimicrobial drugs explored by Atomic Force Microscopy. Semin. Cell Develop. Biol. 2018, 73, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Hanif, B.; Jamil, N.; Shah, M.R. Surface topological differences of phage infected uropathogenic Escherichia coli (UPEC) strains, revealed by atomic force microscopy. SpringerPlus 2016, 5, 2112. [Google Scholar] [CrossRef]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef]
- Cummings, R.D. Stuck on sugars—How carbohydrates regulate cell adhesion, recognition, and signaling. Glycoconj. J. 2019, 36, 241–257. [Google Scholar] [CrossRef]
- Foster, T.J. The MSCRAMM family of cell-wall-anchored surface proteins of Gram-positive cocci. Trends Microbiol. 2019, in press. [Google Scholar] [CrossRef]
- Hewelt-Belka, W.; Nakonieczna, J.; Belka, M.; Bączek, T.; Namieśnik, J.; Kot-Wasik, A. Untargeted lipidomics reveals differences in the lipid pattern among clinical isolates of Staphylococcus aureus resistant and sensitive to antibiotics. J. Proteome Res. 2016, 15, 914–922. [Google Scholar] [CrossRef]
- Kilelee, E.; Pokorny, A.; Yeaman, M.R.; Bayer, A.S. Lysyl-phosphatidylglycerol attenuates membrane perturbation rather than surface association of the cationic antimicrobial peptide 6W-RP-1 in a model membrane system: Implications for daptomycin resistance. Antimicrob. Agents Chemoth. 2010, 54, 4476–4479. [Google Scholar] [CrossRef]
- Sen, S.; Sirobhushanam, S.; Johnson, S.R.; Song, Y.; Tefft, R.; Gatto, C.; Wilkinson, B.J. Growth-environment dependent modulation of Staphylococcus aureus branched-chain to straight-chain fatty acid ratio and incorporation of unsaturated fatty acids. PLoS ONE 2016, 11, e0165300. [Google Scholar] [CrossRef]
- Carniello, V.; Harapanahalli, A.K.; Busscher, H.J.; van der Mei, H.C. Adhesion force sensing and activation of a membrane-bound sensor to activate nisin efflux pumps in Staphylococcus aureus under mechanical and chemical stresses. J. Colloid Interf. Sci. 2018, 512, 14–20. [Google Scholar] [CrossRef]
- Esteve-Pastor, M.A.; Hernández-Romero, D.; Valdés, M.; Marín, F. New approaches to the role of thrombin in acute coronary syndromes: Quo vadis bivalirudin, a direct thrombin inhibitor? Molecules 2016, 21, 284. [Google Scholar] [CrossRef]
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Hosseini, E.; Ghasemzadeh, M.; Azizvakili, E.; Beshkar, P. Platelet spreading on fibrinogen matrix, a reliable and sensitive marker of platelet functional activity during storage. J. Thromb. Thrombolysis. 2019, 1–9. [Google Scholar] [CrossRef]
- Kerrigan, S.W.; Clarke, N.; Loughman, A.; Meade, G.; Foster, T.J.; Cox, D. Molecular basis for Staphylococcus aureus-mediated platelet aggregate formation under arterial shear in vitro. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 335–340. [Google Scholar] [CrossRef]
- Zarka, R.; Horev, M.B.; Volberg, T.; Neubauer, S.; Kessler, H.; Spatz, J.P.; Geiger, B. Differential modulation of platelet adhesion and spreading by adhesive ligand density. Nano Lett. 2019, 19, 1418–1427. [Google Scholar] [CrossRef]
- McEwen, B.J. The influence of diet and nutrients on platelet function. Semin. Thromb. Hemost. 2014, 40, 214–226. [Google Scholar] [CrossRef]
- Olas, B. Dietary supplements with antiplatelet activity: A solution for everyone? Adv. Nutr. 2018, 9, 51–57. [Google Scholar] [CrossRef]
- Wright, B.; Spencer, J.P.; Lovegrove, J.A.; Gibbins, J.M. Flavonoid inhibitory pharmacodynamics on platelet function in physiological environments. Food Funct. 2013, 4, 1803–1810. [Google Scholar] [CrossRef]
- Malinowska, J.; Oleszek, W.; Stochmal, A.; Olas, B. The polyphenol-rich extracts from black chokeberry and grape seeds impair changes in the platelet adhesion and aggregation induced by a model of hyperhomocysteinemia. Eur. J. Nutr. 2013, 52, 1049–1057. [Google Scholar] [CrossRef]
- Rahman, K.; Lowe, G.M.; Smith, S. Aged garlic extract inhibits human platelet aggregation by altering intracellular signaling and platelet shape change. J. Nutr. 2016, 146, 410S–415S. [Google Scholar] [CrossRef]
- Ellingsen, I.; Hjerkinn, E.M.; Seljeflot, I.; Arnesen, H.; Tonstad, S. Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men. British J. Nutr. 2008, 99, 674–681. [Google Scholar] [CrossRef] [Green Version]
- O’Kennedy, N.; Raederstorff, D.; Duttaroy, A.K. Fruitflow®: The first European Food Safety Authority-approved natural cardio-protective functional ingredient. Eur. J. Nutr. 2017, 56, 461–482. [Google Scholar] [CrossRef]
- Laskowski, D.; Strzelecki, J.; Pawlak, K.; Dahm, H.; Balter, A. Effect of ampicillin on adhesive properties of bacteria examined by atomic force microscopy. Micron 2018, 112, 84–90. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of LCE are not available. |









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, B.; Laskowski, D.; Bernat, P.; Micota, B.; Więckowska-Szakiel, M.; Podsędek, A.; Różalska, B. Molecular Mechanisms of Leonurus Cardiaca L. Extract Activity in Prevention of Staphylococcal Endocarditis—Study on in Vitro and ex Vivo Models. Molecules 2019, 24, 3318. https://doi.org/10.3390/molecules24183318
Sadowska B, Laskowski D, Bernat P, Micota B, Więckowska-Szakiel M, Podsędek A, Różalska B. Molecular Mechanisms of Leonurus Cardiaca L. Extract Activity in Prevention of Staphylococcal Endocarditis—Study on in Vitro and ex Vivo Models. Molecules. 2019; 24(18):3318. https://doi.org/10.3390/molecules24183318
Chicago/Turabian StyleSadowska, Beata, Dariusz Laskowski, Przemysław Bernat, Bartłomiej Micota, Marzena Więckowska-Szakiel, Anna Podsędek, and Barbara Różalska. 2019. "Molecular Mechanisms of Leonurus Cardiaca L. Extract Activity in Prevention of Staphylococcal Endocarditis—Study on in Vitro and ex Vivo Models" Molecules 24, no. 18: 3318. https://doi.org/10.3390/molecules24183318
APA StyleSadowska, B., Laskowski, D., Bernat, P., Micota, B., Więckowska-Szakiel, M., Podsędek, A., & Różalska, B. (2019). Molecular Mechanisms of Leonurus Cardiaca L. Extract Activity in Prevention of Staphylococcal Endocarditis—Study on in Vitro and ex Vivo Models. Molecules, 24(18), 3318. https://doi.org/10.3390/molecules24183318