Effect of Nanocrystallization of Anthocyanins Extracted from Two Types of Red-Fleshed Apple Varieties on Its Stability and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Anthocyanins and Total Phenols Contents of the Two Types of Red-fleshed Apple
2.2. The Comparison of Encapsulation Properties of the Two Types of Red-fleshed Apple using CSNPs
2.2.1. The Effect of Different Anthocyanin Concentrations on CSNPs Encapsulation
2.2.2. The Effect of Different Loading Times on CSNPs Encapsulation
2.3. The Effect of Different Anthocyanins Concentrations and Encapsulating Times on Anthocyanins Corn Starch Hybrid Nanoparticles
2.4. Transmission Electron Microscopy Images of Anthocyanin Corn Starch Hybrid Nanoparticles
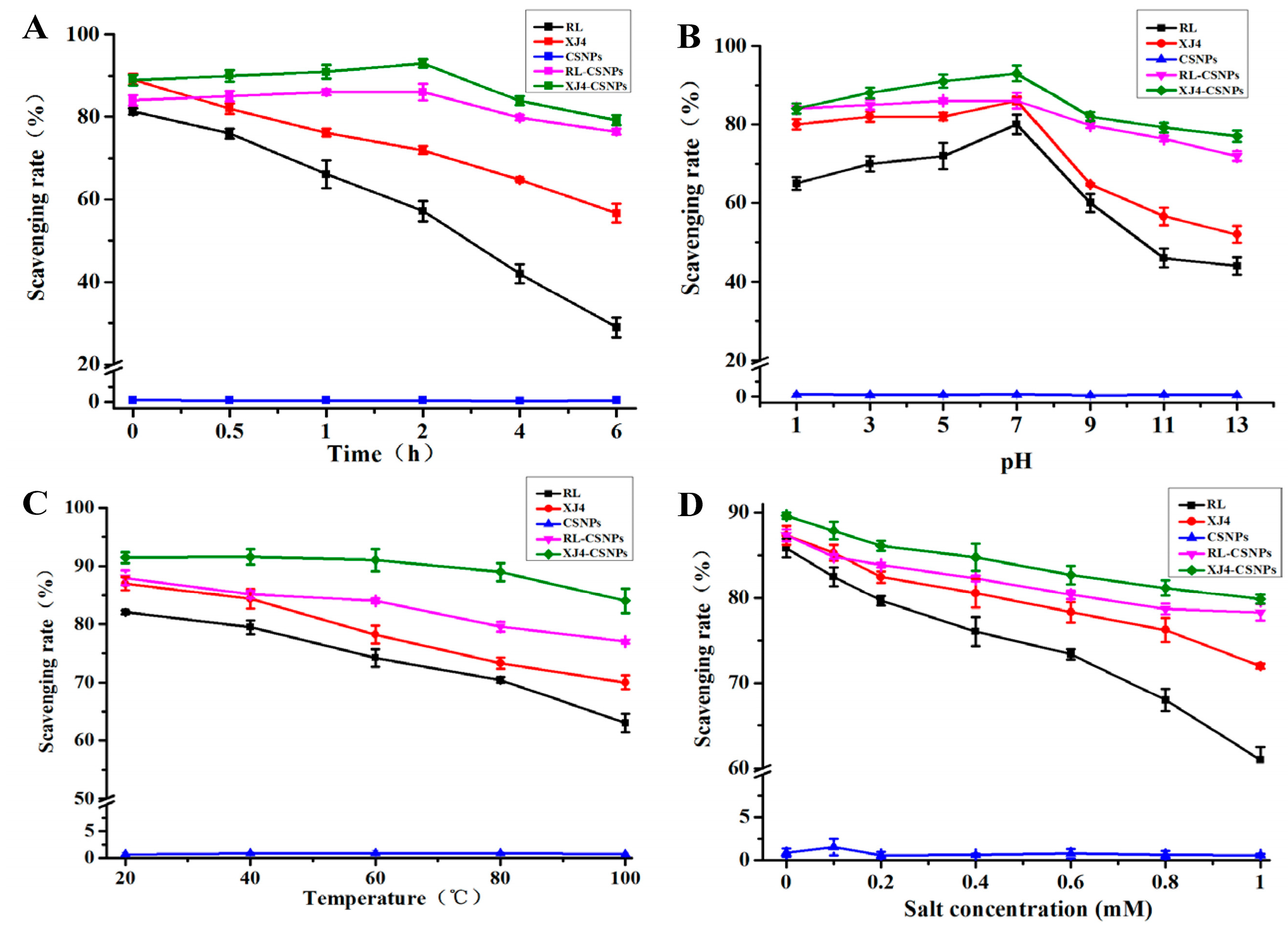
2.5. The Stability of RL-CSNPs and XJ4-CSNPs under Different Stress Treatments
2.5.1. The Effect of UV Light
2.5.2. The Effect of pH
2.5.3. The Effect of Temperature
2.5.4. The Effect of Salinity
2.6. The Antioxidant Activity of RL-CSNPs and XJ4-CSNPs under Different Stress Treatments
2.7. The in Vitro Release Rate of Anthocynins after Nanocrystallization
3. Material and Methods
3.1. Materials
3.2. Measurement of Total Anthocyanins and Total Phenols Content
3.3. Preparation of CSNPs
3.4. Preparation of RL-CSNPs and XJ4-CSNPs
3.5. Measurement of Particles Size and PDI
3.6. Transmission Electron Microscopy (TEM)
3.7. Stress Treatments of RL-CSNPs and XJ4-CSNPs
3.8. Measurement of Scavenging Capacity
3.9. Measurement of in Vitro Release Rate of Anthocyanins
3.10. Sample Preparation, Metabolite Identification and Quantification
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Garanto, X.; Badiella, L.; Motilva, M.-J. Seasonal variability of the phytochemical composition of new red-fleshed apple varieties compared with traditional and new white-fleshed varieties. J. Agric. Food Chem. 2018, 66, 10011–10025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Zhao, R.X.; Liu, W.L.; Sun, X.H.; Bai, S.H.; Xiang, Y.; Dai, H.Y. The anthocyanins component and the influence factors of contents in red flesh apple ‘Hong-XunNo.1’. Eur. J. Hortic. Sci. 2016, 81, 248–254. [Google Scholar] [CrossRef]
- Gu, K.D.; Wang, C.K.; Hu, D.G.; Hao, Y.J. How do anthocyanins paint our horticultural products. Sci. Hortic. 2019, 249, 257–262. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhu, J.; Dai, H.Y. Morphological characteristics and pollination compatibility of a new red flesh apple, Hongxun No.1. Res. Crop. 2013, 14, 199–204. [Google Scholar]
- Xiang, Y.; Zhao, R.X.; Lai, F.N.; Sun, X.; Sun, X.H.; Dai, H.Y.; Zhang, Y.G. Analysis of flavonoid components and antioxidant activity in peel of red-fleshed apple. Plant. Physiol. 2016, 52, 1353–1360. [Google Scholar]
- Clifford, M.N. Anthocyanins-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Liang, C.; Xin, X.; Yuan, Q.; Su, D.; Wei, L. Phytochemical properties and antioxidant capacities of various colored berries. J. Sci. Food Agric. 2014, 94, 180–188. [Google Scholar]
- Sousa, A.; Araújo, P.; Azevedo, J.; Cruz, L.; Fernandes, I.; Mateus, N. DeFreitas, V. Antioxidant and antiproliferative properties of 3-deoxyanthocyanidins. Food Chem. 2016, 192, 142–148. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, S.; Zhang, Z.; Fang, H.; Xu, H.; Wang, Y.; Chen, X. Malus sieversii: The origin, flavonoid synthesis mechanism, and breeding of red-skinned and red-fleshed apples. Hortic. Res. 2018, 5, 70. [Google Scholar] [CrossRef]
- Charepalli, V.; Reddivari, L.; Vadde, R.; Walia, S.; Radhakrishnan, S.; Vanamala, J.K.P. Eugenia jambolana (Java Plum) fruit extract exhibits anti-cancer activity against early stage human HTC-116 colon cancer cells andcolon cancer stem cells. Cancers 2016, 8, 29. [Google Scholar] [CrossRef]
- Li, A.L.; Ru, Z.L. Study on ultrasonic extraction method of red pigment in pyracantha fortuneana. Food Res. Dev. 2008, 29, 11–14. [Google Scholar]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.-A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar]
- Xiang, Y.; Lai, F.N.; He, G.F.; Li, Y.P.; Yang, L.L.; Shen, W.; Huo, H.Q.; Zhu, J.; Dai, H.Y.; Zhang, Y.G. Alleviation of rosup-induced oxidative stress in porcine granulosa cells by anthocyanins from red-fleshed apples. PLoS ONE 2017, 12, e-0184033. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Shiga, K.; Ohshima, K.; Kawakishi, S.; Osawa, T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem. Pharmacol. 1996, 52, 1033–1039. [Google Scholar] [CrossRef]
- Xie, Y.L.; Li, X.C.D.; Chen, J.Y.; Deng, Y.M.; Lu, W.B.; Chen, D.F. pH effect and chemical mechanisms of antioxidant higenamine. Molecules 2018, 23, 2176. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Yang, K.M.; Chiang, P.Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huo, H.Q.; Sun, X.H.; Zhu, J.; Dai, H.Y.; Zhang, Y.G. Nanocrystallization of anthocyanin extract from red-fleshed apple ′QN-5′ improved its antioxidant effect through enhanced stability and activity under stressful conditions. Molecules 2019, 24, 1421. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, C.Z.; Jiang, S.S.; Xiong, L.; Sun, Q.J. Characterization of starch nanoparticles prepared by nanoprecipitation: Influence of amylose content and starch type. Ind. Crop. Prod. 2016, 87, 182–190. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y. Preparation, characterization and evaluation of tea polyphenol–Zn complex loaded β-chitosan nanoparticles. Food Hydrocoll. 2015, 48, 260–273. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.M.; Gu, L.W. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Qiu, C.; Chang, R.R.; Yang, J.; Ge, S.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q.J. Preparation and characterization of essential oil-loaded starch nanoparticles formed by short glucan chains. Food Chem. 2017, 221, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Ge, S.J.; Yang, J.; Xu, Y.Y.; Zhao, M.; Xiong, L.; Sun, Q.J. Adsorption mechanism of polyphenols onto starch nanoparticles and enhanced antioxidant activity under adverse conditions. J. Funct. Foods 2016, 26, 632–644. [Google Scholar] [CrossRef]
- Nocker, V.S.; Berry, G.; Najdowski, J.; Michelutti, R.; Luffman, M.; Forsline, P.; Alsmairat, N.; Beaudry, R.; Nair, M.G.; Ordidge, M. Genetic diversity of red-fleshed apples (Malus). Euphytica 2011, 185, 281–293. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidlry, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Li, M.; Yang, J.; Xiong, L.; Sun, Q.J. Fabrication and characterization of biocompatible hybridnanoparticles from spontaneous co-assembly of casein/gliadin and proanthocyanidin. Food Hydrocoll. 2017, 73, 74–89. [Google Scholar] [CrossRef]
- Cornelia, M.K.; Rainer, H.M. Challenges and solutions for the delivery of biotech drugs-a review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1–F2. [Google Scholar] [CrossRef]
- Cai, W.G.; Wu, W.; Shao, J.F.; Chen, Q.; Wang, Y.B.; Liu, Z.Q. Determination of polyphenol content in Houttuynia cordata Thunb. By Folin-Ciocalteu colorimetric method. Food Science 2010, 31, 201–204. [Google Scholar]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Vainstein, A.; Chen, S.; Ma, H. Regulation of fig (Ficus carica L.) fruit color: Metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway. Frontiers Plant. Science 2017, 8, 1990. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, X.; Liu, S.; Zhao, P.; Huo, H.; Zhang, Y. Effect of Nanocrystallization of Anthocyanins Extracted from Two Types of Red-Fleshed Apple Varieties on Its Stability and Antioxidant Activity. Molecules 2019, 24, 3366. https://doi.org/10.3390/molecules24183366
Xu J, Li X, Liu S, Zhao P, Huo H, Zhang Y. Effect of Nanocrystallization of Anthocyanins Extracted from Two Types of Red-Fleshed Apple Varieties on Its Stability and Antioxidant Activity. Molecules. 2019; 24(18):3366. https://doi.org/10.3390/molecules24183366
Chicago/Turabian StyleXu, Jihua, Xinxin Li, Shifeng Liu, Peilei Zhao, Heqiang Huo, and Yugang Zhang. 2019. "Effect of Nanocrystallization of Anthocyanins Extracted from Two Types of Red-Fleshed Apple Varieties on Its Stability and Antioxidant Activity" Molecules 24, no. 18: 3366. https://doi.org/10.3390/molecules24183366




