Effects of the Ethanol Extract of Dipterocarpus alatus Leaf on the Unpredictable Chronic Mild Stress-Induced Depression in ICR Mice and Its Possible Mechanism of Action
Abstract
:1. Introduction
2. Results
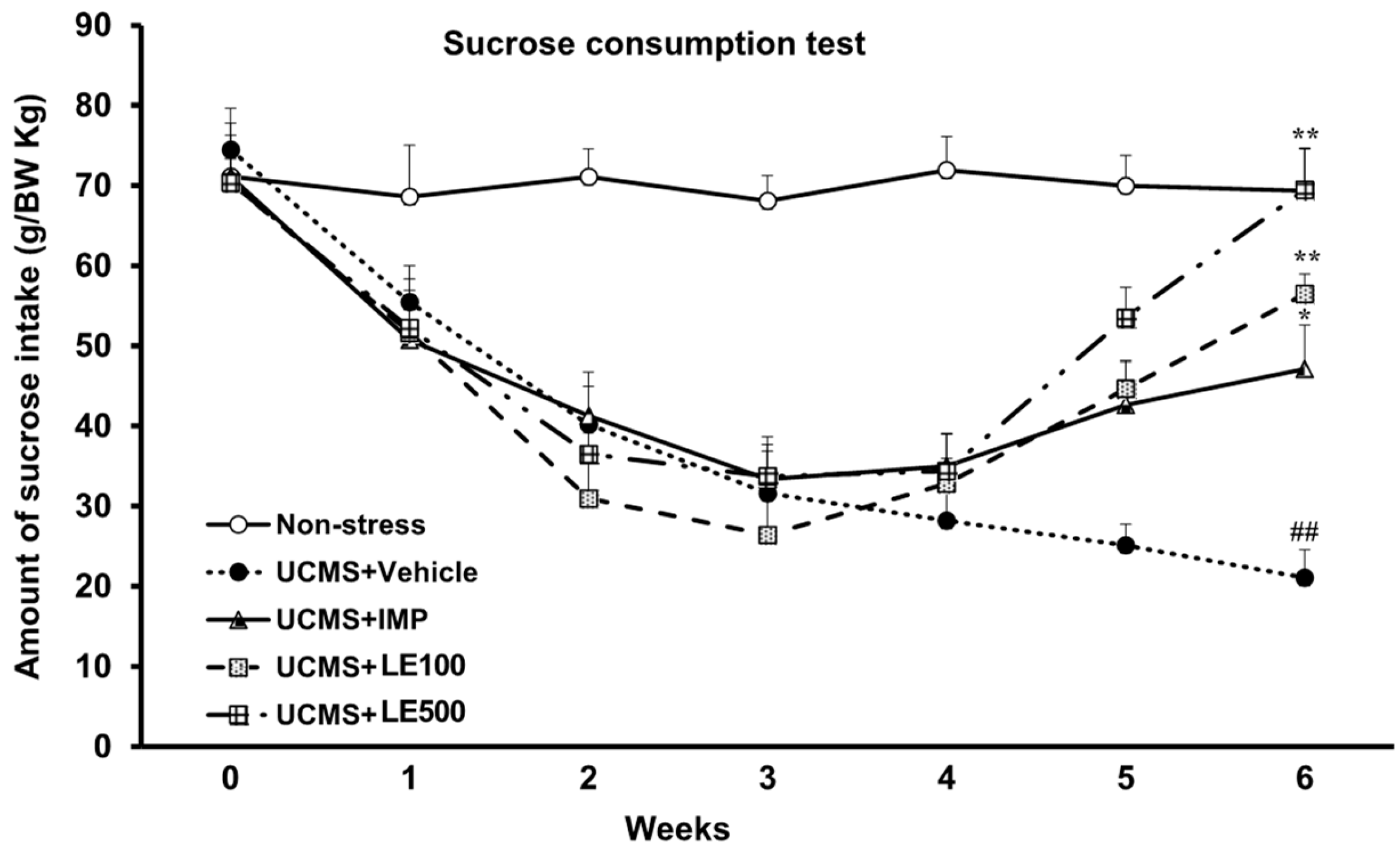
2.1. The Effect of the Ethanol Extract of D. Alatus Leaf (LE) on Anhedonia Behavior Using Sucrose Preference Test
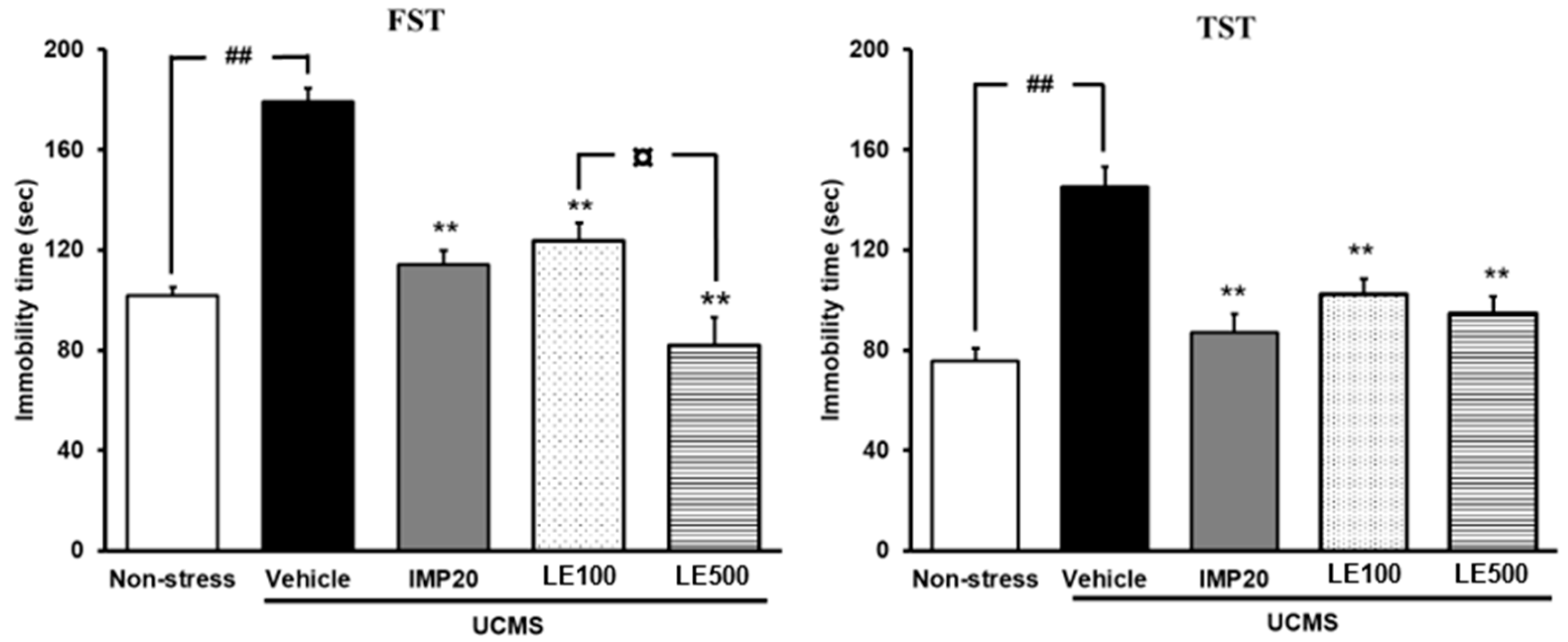
2.2. The Effect of the Ethanol Extract of D. Alatus Leaf (LE) on Hopeless Behavior Using Tail Suspension Test (TST) and Forced Swimming Test (FST)
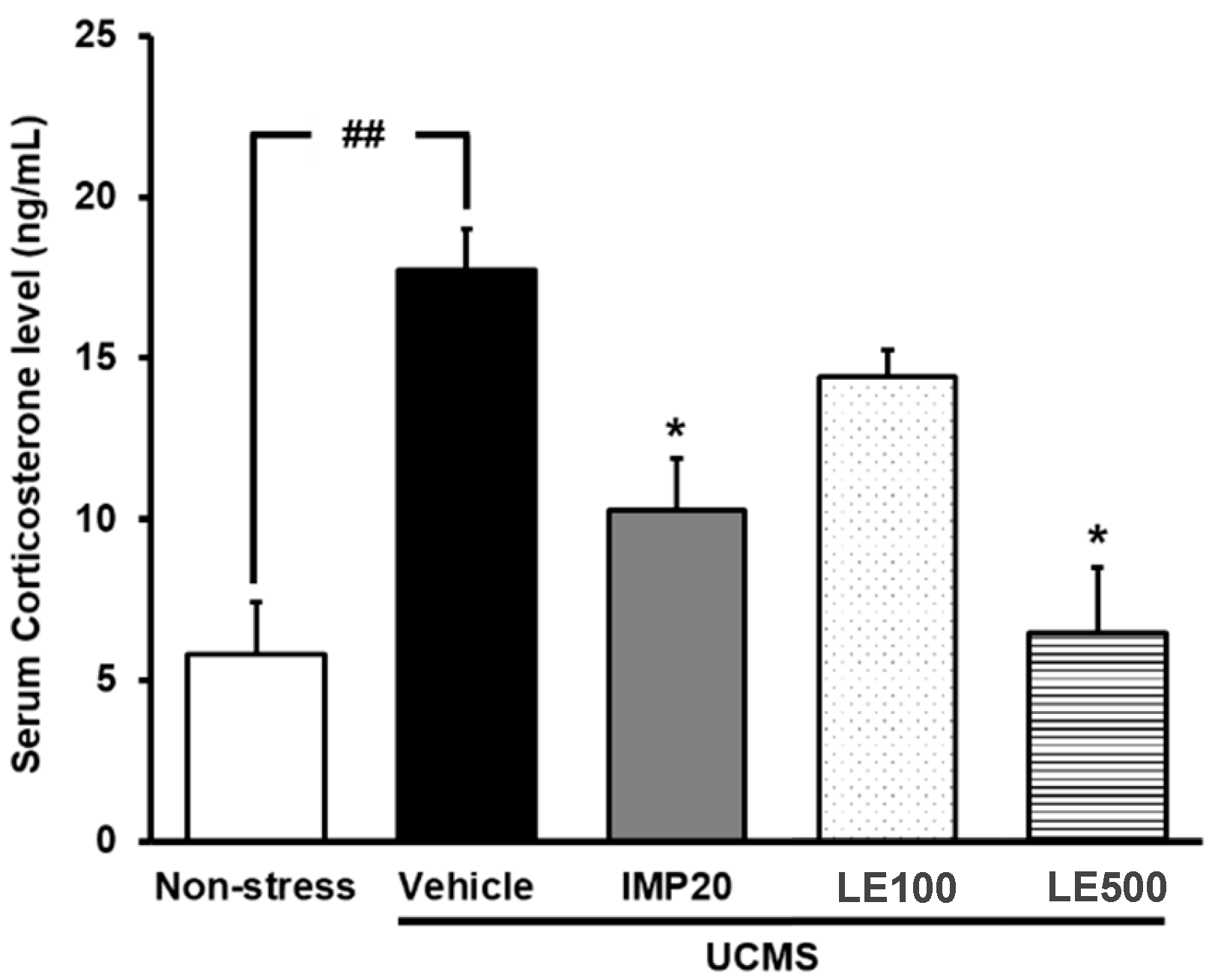
2.3. The Effect of the Ethanol Extract of D. Alatus Leaf (LE) on Serum Corticosterone (CORT) Levels in Unpredictable Chronic Mild Stress (UCMS) Mice
2.4. Effect of and the Ethanol Extract of D. Alatus Leaf (LE) on Unpredictable Chronic Mild Stress (UCMS)-Induced Changes in Serum- and Glucocortoid-Inducible Kinase 1 (SGK1), Cyclic AMP-Responsive Element Binding (CREB) and Brain-Derived Neurotrophic Factor (BDNF) mRNA Expressions in Mice Frontal Cortex and Hippocampus
2.5. Monoamine Oxidase (MAO)-A and MAO-B Inhibitory Activities of the Ethanol Extract of D. alatus Leaf (LE)
2.6. High Performance Liquid Chromatography (HPLC) Analysis of and the Ethanol Extract of D. alatus Leaf (LE) and Method of Validation
3. Discussion
4. Material and Methods
4.1. Preparation of the Leaf Extract of Dipterocarpus alatus
4.2. Animals
4.3. Unpredictable Chronic Mild Stress (UCMS)
4.4. Drug Administration
4.5. Sucrose Preference Test
4.6. Tail Suspension Test (TST)
4.7. Forced Swimming Test (FST)
4.8. Locomotor Test
4.9. Serum Corticosterone Assay
4.10. Quantitative Real Time PCR (QPCR)
4.11. Human Monoamine Oxidase (MAO)-A and MAO-B Inhibitory Activity Assay
4.12. High-Performance Liquid Chromatography (HPLC) Analysis and Validation of Analytical Method
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Keane, V.; Frodl, T.; Dinan, T.G. A review of atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology 2012, 37, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Solomon, M.B.; Carvalho-Netto, E.; Myers, B. Neural regulation of the stress response: Glucocorticoid feedback mechanisms. Braz. J. Med. Biol. Res. 2012, 45, 292–298. [Google Scholar] [CrossRef]
- Odaka, H.; Adachi, N.; Numakawa, T. Impact of glucocorticoid on neurogenesis. Neural Regen. Res. 2017, 12, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Kojima, M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017, 97, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Strutz-Seebohm, N.; Seebohm, G.; Lang, U.E. Significance of SGK1 in the regulation of neuronal function. J. Physiol. 2010, 588 Pt 18, 3349–3354. [Google Scholar] [CrossRef]
- Anacker, C.; Cattaneo, A.; Musaelyan, K.; Zunszain, P.A.; Horowitz, M.; Molteni, R.; Luoni, A.; Calabrese, F.; Tansey, K.; Gennarelli, M.; et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 8708–8713. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Xu, Y.; Zhao, Z.; Wu, X.; Du, Y.; Sun, J.; Yi, T.; Dong, J.; Liu, B. Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int. J. Mol. Med. 2016, 38, 337–344. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, X.; Wang, F.; Ma, J.; Su, G.; Dong, Y.; Yang, J.; Wu, C. Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression. Sci. Rep. 2016, 6, 30951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnick, C.R.; Hocking, G.M. Ethnobotanical records of drug plants described in Valmiki Ramayana and their uses in the Ayuvedic system of medicine. Q. J. Crude Drug Res. 1975, 13, 143–154. [Google Scholar] [CrossRef]
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S. A Phytochemical, ethnomedicinal and pharmacological review of genus Dipterocarpus. Int. J. Pharm. Pharm. Sci. 2015, 7, 27–38. [Google Scholar]
- Chen, C.J.; Jiang, R.; Wang, G.; Jiao, R.H.; Tancharoen, C.; Sudto, K.; Vajarothai, S.; Hannongbua, S.; Ge, H.M.; Tan, R.X. Oligostilbenoids with acetylcholinesterase inhibitory activity from Dipterocarpus alatus. Planta Med. 2014, 80, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Yongram, C.; Sungthong, B.; Puthongking, P.; Weerapreeyakul, N. Activities of leaves, bark, twigs and oleo-resin of Dipterocarpus alatus. Molecules 2019, 24, 3083. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K. Leaf flavonoid patterns in Dipterocarpus and Hopea (Dipterocarpaceae). Bot. J. Linn. Soc. 2003, 143, 43–46. [Google Scholar] [CrossRef]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, W.; Peng, Y.; Deng, Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab. Brain Dis. 2019, 34, 485–494. [Google Scholar] [CrossRef]
- Cheng, H.C. The power issue: Determination of KB or Ki from IC50: A closer look at the Cheng–Prusoff equation, the Schild plot and related power equations. J. Pharmacol. Toxicol. Methods 2001, 46, 61–71. [Google Scholar] [CrossRef]
- Manley-King, C.I.; Bergh, J.J.; Petzer, J.P. Inhibition of monoamine oxidase by C5-substituted phthalimide analogues. Bioorg. Med. Chem. 2011, 19, 4829–4840. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2016, 6, 78–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Dong, Y.; Su, Q.; Wang, H.; Chen, Y.; Xue, W.; Chen, C.; Xia, B.; Duan, J.; Chen, G. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav. Brain Res. 2016, 308, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Zu, X.; Zhang, M.; Li, W.; Xie, H.; Lin, Z.; Yang, N.; Liu, X.; Zhang, W. Antidepressant-like effect of bacopaside I in mice exposed to chronic unpredictable mild stress by modulating the hypothalamic-pituitary-adrenal axis function and activating BDNF signaling pathway. Neurochem. Res. 2017, 42, 3233–3244. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Cattaneo, A.; Riva, M.A. Stress-induced mechanisms in mental illness: A role for glucocorticoid signalling. J. Steroid Biochem. Mol. Biol. 2016, 160, 169–174. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, Z.; Wang, H.; Xue, F.; Chen, Y.; Wang, Y.; Wang, H.; Tan, Q. Gastrodin ameliorates depressive-like behaviors and up-regulates the expression of BDNF in the hippocampus and hippocampal-derived astrocyte of rats. Neurochem. Res. 2014, 39, 172–179. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, L.; Ji, Z.; Li, X.; Zhang, C.; Ma, Z.; Fu, Q.; Qu, R.; Ma, S. Radix Scutellariae attenuates CUMS-Induced depressive-like behavior by promoting neurogenesis via cAMP/PKA pathway. Neurochem. Res. 2018, 43, 2111–2120. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: From neurotransmitter imbalance to impaired neurogenesis. J. Neural Transm. 2018, 125, 53. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Li, X. Discovery of monoamine oxidase inhibitors by medicinal chemistry approaches. MedChemComm 2019, 10, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Ishisaka, M.; Kakefuda, K.; Yamauchi, M.; Tsuruma, K.; Shimazawa, M.; Tsuruta, A.; Hara, H. Luteolin shows an antidepressant-like effect via suppressing endoplasmic reticulum stress. Biol. Pharm. Bull. 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lei, S.; Zhou, S.; Jin, L.; Zeng, S.; Jiang, H.; Zhou, H. Luteolin shows antidepressant-like effect by inhibiting and downregulating plasma membrane monoamine transporter (PMAT, Slc29a4). J. Funct. Foods 2019, 54, 440–448. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, Y.B.; Han, P.L.; Lee, J.K.; Suh, H.W. Antidepressant-like effect of kaempferol and quercitirin, isolated from Opuntia ficus-indica var. saboten. Exp. Neurobiol. 2010, 19, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A.; Mehta, V.; Udayabanu, M. Rutin alleviates chronic unpredictable stress-induced behavioral alterations and hippocampal damage in mice. Neurosci. Lett. 2017, 656, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Iwata, N.; Ferdousi, F.; Isoda, H. Antidepressant-like effect of ferulic acid via promotion of energy metabolism activity. Mol. Nutr. Food Res. 2019, e1900327. [Google Scholar] [CrossRef]
- Mizuki, D.; Matsumoto, K.; Tanaka, K.; Le, X.T.; Fujiwara, H.; Ishikawa, T.; Higuchi, Y. Antidepressant-like effect of Butea superba in mice exposed to chronic mild stress and its possible mechanism of action. J. Ethnopharmacol. 2014, 156, 16–25. [Google Scholar] [CrossRef]
- Chatterjee, M.; Jaiswal, M.; Palit, G. Comparative evaluation of forced swim test and tail suspension test as models of negative symptom of schizophrenia in rodents. ISRN Psychiatry 2012, 12, 595141. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Khamphukdee, C.; Monthakantirat, O.; Chulikhit, Y.; Buttachon, S.; Lee, M.; Silva, A.M.S.; Sekeroglu, N.; Kijjoa, A. Chemical constituents and antidepressant-like effects in ovariectomized mice of the ethanol extract of Alternanthera philoxeroides. Molecules 2018, 23, 2202. [Google Scholar] [CrossRef] [PubMed]
- Khamphukdee, C.; Chulikhit, Y.; Daodee, S.; Monthakantirat, O. Potential of Alternanthera philoxeroides on improvement of anxiety-like behavior induced by ovariectomized mice model. Indian J. Pharm. Educ. 2017, 51, S494–S497. [Google Scholar] [CrossRef]
- Shabir, G.A.; Lough, W.J.; Arain, S.A.; Bradshaw, T.K. Evaluation and application of best practice in analytical method validation. J. Liquid Chromatogr. Relat. Technol. 2007, 30, 311–333. [Google Scholar] [CrossRef]
Sample Availability: Samples of the crude ethanol extract are available from the authors. |



| Extract/Compounds | IC50 (μM) | Ki (μM) | Si | |||
|---|---|---|---|---|---|---|
| MAO-A | MAO-B | MAO-A | MAO-B | MAO-A | MAO-B | |
| D. alatus leaf extract (LE) | 197.70 ± 0.07 µg/mL | 1158.00 ± 0.05 µg/mL | 52.09 | 498.80 | 0.10 | 0.09.58 |
| Clorgyline * | 0.14 ± 0.04 | 1.40 ± 0.24 | 0.037 | 0.603 | 0.061 | 16.276 |
| Deprenyl ** | 950.20 ± 0.14 | 0.21 ± 0.08 | 250.380 | 0.087 | 2869.095 | 0.000348 |
| Gene | Primer Sequence | Fragment Size |
|---|---|---|
| SGK1 | Forward: 5′-GGG TGC CAA GGA TGA CTT TA-3′ Reverse: 5′-CTC GGT AAA CTC GGG ATC GA-3′ | 154 bp |
| CREB | Forward: 5′-TAC CCA GGG AGG AGC AAT AC-3′ Reverse: 5′-GAG GCA GCT TGA ACA ACA AC-3′ | 183 bp |
| BDNF | Forward: 5′-GAC AAG GCA ACT TGG CCT AC-3′ Reverse: 5′-CCT GTC ACA CAC GCT CAG CTC-3′ | 334 bp |
| GAPDH | Forward: 5’-ACC ACA GTC CAT GCC ATC AC-3’ Reverse: 5’-TCC ACC ACC CTG TTG CTG TA-3’ | 452 bp |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daodee, S.; Monthakantirat, O.; Ruengwinitwong, K.; Gatenakorn, K.; Maneenet, J.; Khamphukdee, C.; Sekeroglu, N.; Chulikhit, Y.; Kijjoa, A. Effects of the Ethanol Extract of Dipterocarpus alatus Leaf on the Unpredictable Chronic Mild Stress-Induced Depression in ICR Mice and Its Possible Mechanism of Action. Molecules 2019, 24, 3396. https://doi.org/10.3390/molecules24183396
Daodee S, Monthakantirat O, Ruengwinitwong K, Gatenakorn K, Maneenet J, Khamphukdee C, Sekeroglu N, Chulikhit Y, Kijjoa A. Effects of the Ethanol Extract of Dipterocarpus alatus Leaf on the Unpredictable Chronic Mild Stress-Induced Depression in ICR Mice and Its Possible Mechanism of Action. Molecules. 2019; 24(18):3396. https://doi.org/10.3390/molecules24183396
Chicago/Turabian StyleDaodee, Supawadee, Orawan Monthakantirat, Kanlaya Ruengwinitwong, Kankrittanon Gatenakorn, Juthamart Maneenet, Charinya Khamphukdee, Nazim Sekeroglu, Yaowared Chulikhit, and Anake Kijjoa. 2019. "Effects of the Ethanol Extract of Dipterocarpus alatus Leaf on the Unpredictable Chronic Mild Stress-Induced Depression in ICR Mice and Its Possible Mechanism of Action" Molecules 24, no. 18: 3396. https://doi.org/10.3390/molecules24183396
APA StyleDaodee, S., Monthakantirat, O., Ruengwinitwong, K., Gatenakorn, K., Maneenet, J., Khamphukdee, C., Sekeroglu, N., Chulikhit, Y., & Kijjoa, A. (2019). Effects of the Ethanol Extract of Dipterocarpus alatus Leaf on the Unpredictable Chronic Mild Stress-Induced Depression in ICR Mice and Its Possible Mechanism of Action. Molecules, 24(18), 3396. https://doi.org/10.3390/molecules24183396










