Selectively Charged and Zwitterionic Analogues of the Smallest Immunogenic Structure of Streptococcus Pneumoniae Type 14
Abstract
:1. Introduction
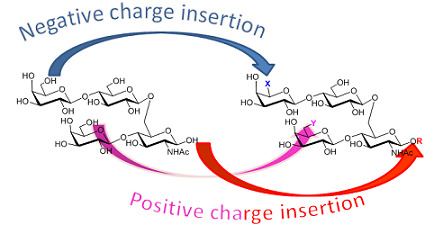
2. Results and Discussion
2.1. Chemistry
2.1.1. Preparation of Required N-acetyl-glucosamine Acceptors 20–23 and Lacturonic Donor 28
2.1.2. Synthesis of the Target Tetrasaccharides 2–7
2.2. Biological Tests
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure for the 6-O-glycosylation: Synthesis of the Tetrasaccharides 42–47
3.1.3. Synthesis of 3-Azidopropyl 2-acetamido-4-O-β-d-galactopyranosyl-6-O-[4-O-(β-d-galactopyranosyl)-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside (48)
3.1.4. Synthesis of Methyl 2-acetamido-3-O-benzyl-4-O-(6-azido-6-deoxy-β-d-galactopyranosyl)-6-O-[4-O-(β-d-galactopyranosyl)-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside (49)
3.1.5. Synthesis of Methyl 2-acetamido-4-O-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl)-6-O-[4-O-(3,4-di-O-acetyl-β-d-galactopyranoyl uronic acid)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside (50)
3.1.6. Synthesis of 3-Ammoniumpropyl 2-acetamido-4-O-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl)-6-O-[4-O-(3,4-di-O-acetyl-2-benzyl-β-d-galactopyranosyl uronate)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside (51)
3.1.7. Synthesis of Methyl 2-acetamido-4-O-(6-ammonium-2,3,4-tri-O-acetyl-6-deoxy-β-d-galactopyranosyl)-6-O-[4-O-(3,4-di-O-acetyl-β-d-galactopyranosyl uronate)-2,3,6-tri-O-acetyl-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside (52).
3.1.8. Synthesis of Methyl 2-acetamido-4-O-β-d-galactopyranosyl-6-O-[4-O-(β-d-galactopyranosyl)-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside (2)
3.1.9. Synthesis of Methyl 2-acetamido-4-O-β-d-galactopyranosyl-6-O-[4-O-(β-d-galactopyranosyl uronate)-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside Ammonium Salt (3)
3.1.10. Synthesis of 3-Aminopropyl 2-acetamido-4-O-β-d-galactopyranosyl-6-O-[4-O-(β-d-galactopyranosyl)-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside Hydrochloride (4)
3.1.11. Synthesis of 3-Ammoniumpropyl 2-acetamido-4-O-β-d-galactopyranosyl-6-O-[4-O-(β-d-galactopyranosyl uronate)-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside (5)
3.1.12. Synthesis of Methyl 2-acetamido-4-O-(6-amino-6-deoxy-β-d-galactopyranosyl)-6-O-[4-O-(β-d-galactopyranosyl)-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside Hydrochloride (6)
3.1.13. Synthesis of Methyl 2-acetamido-4-O-(6-ammonium-6-deoxy-β-d-galactopyranosyl)-6-O-[4-O-(β-d-galactopyranosyl uronate)-β-d-glucopyranosyl]-2-deoxy-β-d-glucopyranoside (7)
3.2. Biological Methods
3.2.1. Test ELISA
3.2.2. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verez-Bencomo, V.; Fernández-Santana, V.; Hardy, E.; Toledo, M.E.; Rodríguez, M.C.; Heynngnezz, L.; Rodriguez, A.; Baly, A.; Herrera, L.; Izquierdo, M.; et al. A Synthetic Conjugate Polysaccharide Vaccine Against Haemophilus influenzae Type b. Science 2004, 305, 522–525. [Google Scholar] [CrossRef]
- Schumann, B.; Anish, C.; Pereira, C.L.; Seeberger, P.H. Carbohydrate Vaccines. In Biotherapeutics: Recent Developments using Chemical and Molecular Biology; Jones, L., McKnight, A.J., Eds.; RSC Drug Discovery Series No. 36. ed. RSC: Cambridge, UK, 2013; pp. 68–104. [Google Scholar]
- Micoli, F.; Costantino, P.; Adamo, R. Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef] [Green Version]
- Colombo, C.; Pitirollo, O.; Lay, L. Recent Advances in the Synthesis of Glycoconjugates for Vaccine Development. Molecules 2018, 23, 1712. [Google Scholar] [CrossRef]
- Nishat, S.; Andreana, P.R. Entirely Carbohydrate-Based Vaccines: An Emerging Field for Specific and Selective Immune Responses. Vaccines 2016, 4, 19. [Google Scholar] [CrossRef]
- Mondiale de la Santé O, World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper–February 2019─Vaccins antipneumococciques conjugués chez les nourrissons et les enfants de moins de 5 ans: note de synthèse de l’OMS–février 2019. Weekly Epidemiological Record=Relevé épidémiologique hebdomadaire 2019, 94, 85–103. [Google Scholar]
- Tzianabos, A.O.; Onderdonk, A.; Rosner, B.; Cisneros, R.; Kasper, D.L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science 1993, 262, 416–419. [Google Scholar] [CrossRef]
- Cobb, B.A.; Wang, Q.; Tzianabos, A.O.; Kasper, D.L. Polysaccharide processing and presentation by the MHCII pathway. Cell 2004, 117, 677–687. [Google Scholar] [CrossRef]
- Van der Put, R.M.F.; Kim, T.H.; Guerreiro, C.; Thouron, F.; Hoogerhout, P.; Sansonetti, P.J.; Westdijk, J.; Stork, M.; Phalipon, A.; Mulard, L.A. A Synthetic Carbohydrate Conjugate Vaccine Candidate against Shigellosis: Improved Bioconjugation and Impact of Alum on Immunogenicity. Bioconjug. Chem. 2016, 27, 883–892. [Google Scholar] [CrossRef]
- Hu, Z.; Bongat White, A.F.; Mulard, L.A. Efficient Iterative Synthesis of O-Acetylated Tri- to Pentadecasaccharides Related to the Lipopolysaccharide of Shigella flexneri Type 3 a through Di- and Trisaccharide Glycosyl Donors. Chem. Asian J. 2017, 12, 419–439. [Google Scholar] [CrossRef]
- Carboni, F.; Adamo, R.; Fabbrini, M.; De Ricco, R.; Cattaneo, V.; Brogioni, B.; Veggi, D.; Pinto, V.; Passalacqua, I.; Oldrini, D.; et al. Structure of a protective epitope of group B Streptococcus type III capsular polysaccharide. Proc. Natl. Acad. Sci. USA 2017, 114, 5017–5022. [Google Scholar] [CrossRef]
- Guazzelli, L.; Ulc, R.; Rydner, L.; Oscarson, S. A synthetic strategy to xylose-containing thioglycoside tri-and tetrasaccharide building blocks corresponding to Cryptococcus neoformans capsular polysaccharide structures. Org. Biomol. Chem. 2015, 13, 6598–6610. [Google Scholar] [CrossRef]
- Guazzelli, L.; McCabe, O.; Oscarson, S. Synthesis of part structures of Cryptococcus neoformans serotype C capsular polysaccharide. Carbohydr. Res. 2016, 433, 5–13. [Google Scholar] [CrossRef]
- Morelli, L.; Fallarini, S.; Lombardi, G.; Colombo, C.; Lay, L.; Compostella, F. Synthesis and biological evaluation of a trisaccharide repeating unit derivative of Streptococcus pneumoniae 19A capsular polysaccharide. Bioorg. Med. Chem. 2018, 26, 5682–5690. [Google Scholar] [CrossRef]
- Menova, P.; Sella, M.; Sellrie, K.; Pereira, C.L.; Seeberger, P.H. Identification of the Minimal Glycotope of Streptococcus pneumoniae 7F Capsular Polysaccharide using Synthetic Oligosaccharides. Chem.: A Eur. J. 2018, 24, 4181–4187. [Google Scholar] [CrossRef]
- Seeberger, P.H.; Pereira, C.L.; Khan, N.; Xiao, G.; Diago-Navarro, E.; Reppe, K.; Opitz, B.; Fries, B.C.; Witzenrath, M. A Semi-Synthetic Glycoconjugate Vaccine Candidate for Carbapenem-Resistant Klebsiella pneumoniae. Angew. Chem. Int. Ed. 2017, 56, 13973–13978. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Yang, L.; Wei, M.; Yan, W.; Xiong, D.-C.; Ye, X.-S. Synthesis and Antigenic Evaluation of Oligosaccharide Mimics of Vi Antigen from Salmonella typhi. Chem. Eur. J. 2017, 23, 10670–10677. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Wei, M.; Song, C.; Ma, Y.-F.; Zheng, X.-J.; Xiong, D.-C.; Ye, X.-S. Chemical synthesis and biological evaluation of penta- to octa- saccharide fragments of Vi polysaccharide from Salmonella typhi. Org. Chem. Front. 2018, 5, 2179–2188. [Google Scholar] [CrossRef]
- Calloni, I.; Unione, L.; Jimenez-Oses, G.; Corzana, F.; Del Bino, L.; Corrado, A.; Pitirollo, O.; Colombo, C.; Lay, L.; Adamo, R.; et al. The Conformation of the Mannopyranosyl Phosphate Repeating Unit of the Capsular Polysaccharide of Neisseria meningitidis Serogroup A and Its Carba-Mimetic. Eur. J. Org. Chem. 2018, 33, 4548–4555. [Google Scholar] [CrossRef]
- Fallarini, S.; Buzzi, B.; Giovarruscio, S.; Polito, L.; Brogioni, G.; Tontini, M.; Berti, F.; Adamo, R.; Lay, L.; Lombardi, G. A Synthetic Disaccharide Analogue from Neisseria meningitidis A Capsular Polysaccharide Stimulates Immune Cell Responses and Induces Immunoglobulin G (IgG) Production in Mice When Protein-Conjugated. ACS Infect. Dis. 2015, 1, 487–496. [Google Scholar] [CrossRef]
- Fusari, M.; Fallarini, S.; Lombardi, G.; Lay, L. Synthesis of di- and tri-saccharide fragments of Salmonella typhi Vi capsular polysaccharide and their zwitterionic analogues. Bioorg. Med. Chem. 2015, 23, 7439–7447. [Google Scholar] [CrossRef]
- Zhang, Q.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Synthetic zwitterionic polysaccharides. Curr. Opin. Chem. Biol. 2017, 40, 95–101. [Google Scholar] [CrossRef]
- Pfister, H.B.; Paoletti, J.; Poveda, A.; Jimenez-Barbero, J.; Mulard, L.A. Zwitterionic Polysaccharides of Shigella sonnei: Synthetic Study toward a Ready-for-Oligomerization Building Block Made of Two Rare Amino Sugars. Synthesis 2018, 50, 4270–4282. [Google Scholar] [CrossRef]
- Bridy-Pappas, A.E.; Margolis, M.B.; Center, K.J.; Isaacman, D.J. Streptococcus pneumoniae: Description of the Pathogen, Disease Epidemiology, Treatment, and Prevention. Pharmacotherapy 2005, 25, 1193–1212. [Google Scholar] [CrossRef]
- Marchetti, F.; Sottana, F.; La Torre, G. Caratteristiche biologiche e dati clinici disponibili per il nuovo vaccino antipneumococcico Synflorix™ (PHiD-CV). Ital. J. Public Health 2009, 6, s59–s72. [Google Scholar]
- Safari, D.; Dekker, H.A.T.; Joosten, J.A.F.; Michalik, D.; Carvalho de Souza, A.; Adamo, R.; Lahmann, M.; Sundgren, A.; Oscarson, S.; Kamerling, J.P.; et al. Identification of the Smallest Structure Capable of Evoking Opsonophagocytic Antibodies against Streptococcus pneumoniae Type 14. Infect. Immun. 2008, 76, 4615–4623. [Google Scholar] [CrossRef]
- Mawas, F.; Niggemann, J.; Jones, C.; Corbel, M.J.; Karmeling, J.P.; Vliegenthart, J.F.G. Immunogenicity in a Mouse Model of a Conjugate Vaccine Made with a Synthetic Single Repeating Unit of Type 14 Pneumococcal Polysaccharide Coupled to CRM197. Infect. Immun. 2002, 70, 5107–5114. [Google Scholar] [CrossRef] [Green Version]
- Kurbatova, E.A.; Akhmatova, N.K.; Akhmatova, E.A.; Egorova, N.B.; Yastrebova, N.E.; Sukhova, E.V.; Yashunsky, D.V.; Tsvetkov, Y.E.; Gening, M.L.; Nifantiev, N.E. Neoglycoconjugate of Tetrasaccharide Representing One Repeating Unit of the Streptococcus pneumoniae Type 14 Capsular Polysaccharide Induces the Production of Opsonizing IgG1 Antibodies and Possesses the Highest Protective Activity As Compared to Hexa- and Octasaccharide Conjugates. Front. Immunol. 2017, 8, 659. [Google Scholar] [CrossRef]
- Safari, D.; Marradi, M.; Chiodo, F.; Th Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P.; et al. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [CrossRef]
- Vetro, M.; Safari, D.; Fallarini, S.; Salsabila, K.; Lahmann, M.; Penadés, S.; Lay, L.; Marradi, M.; Compostella, F. Preparation and immunogenicity of gold glyco-nanoparticles as antipneumococcal vaccine model. Nanomedicine 2017, 12, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Guazzelli, L.; Catelani, G.; D′Andrea, F. Lactose as an inexpensive starting material for the preparation of aldohexos-5-uloses: Synthesis of l-ribo and d-lyxo derivatives. Carbohydr. Res. 2010, 345, 369–376. [Google Scholar] [CrossRef]
- Bianchini, R.; Rolla, M.; Isaad, J.; Catelani, G.; Guazzelli, L.; Corsi, M.; Bonanni, M. Efficient double glycoconjugation to naturalize high molecular weight disperse dyes. Carbohydr. Res. 2012, 356, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Pistarà, V.; Corsaro, A.; Rescifina, A.; Catelani, G.; D′Andrea, F.; Guazzelli, L. Prevalence of Oxetanose Forms in the Tautomeric Equilibrium of β-Hydroxy-1,5-dicarbonyl Monosaccharides. J. Org. Chem. 2013, 78, 9444–9449. [Google Scholar] [CrossRef]
- Guazzelli, L.; Catelani, G.; D′Andrea, F.; Gragnani, T.; Griselli, A. Stereoselective Access to the β-d-N-Acetylhexosaminyl-(1→4)-1-deoxy-d-nojirimycin Disaccharide Series Avoiding the Glycosylation Reaction. Eur. J. Org. Chem. 2014, 29, 6527–6537. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.V.; Nitz, M. Protecting Group Free Glycosidations Using p-Toluenesulfonohydrazide Donors. Org. Lett. 2008, 10, 3461–3463. [Google Scholar] [CrossRef]
- Willems, M.M.; Zom, G.G.; Meeuwenoord, N.; Ossendorp, F.A.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C.; Filippov, D.V. Design, automated synthesis and immunological evaluation of NOD2-ligand–antigen conjugates. Beilstein, J. Org. Chem. 2014, 10, 1445–1453. [Google Scholar] [CrossRef]
- Yoshino, T.; Reuter, G.; Kelm, S.; Schauer, R. Facile synthesis of 2′-substituted lactoses. Glycoconj. J. 1986, 3, 7–14. [Google Scholar] [CrossRef]
- Barili, P.L.; Catelani, G.; D′Andrea, F.; De Rensis, F.; Falcini, P. Improved preparation of 2,3:5,6:3′,4′-tri-O-isopropylidenelactose dimethyl acetal and its 6′-O-(1-methoxy-1-methylethyl) derivative. Carbohydr. Res. 1997, 298, 75–84. [Google Scholar] [CrossRef]
- Wang, J.-W.; Asnani, A.; Auzanneau, F.-I. Synthesis of a BSA-Lex glycoconjugate and recognition of Lex analogues by the anti-Lex monoclonal antibody SH1: The identification of a non-cross-reactive analogue. Bioorg. Med. Chem. 2010, 18, 7174–7185. [Google Scholar] [CrossRef]
- Wang, A.; Hendel, J.L.; Auzanneau, F.-I. Convergent syntheses of LeX analogues. Beilstein, J. Org. Chem. 2010, 6, 17. [Google Scholar] [CrossRef]
- Debost, J.L.; Gelas, J.; Horton, D. Selective preparation of mono-and diacetals of d-mannitol. J. Org. Chem. 1983, 48, 1381–1382. [Google Scholar] [CrossRef]
- Cheng, H.; Cao, X.; Xian, M.; Fang, L.; Cai, T.B.; Ji, J.J.; Tunac, J.B.; Sun, D.; Wang, P.G. Synthesis and Enzyme-Specific Activation of Carbohydrate-Geldanamycin Conjugates with Potent Anticancer Activity. J. Med. Chem. 2005, 48, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Fais, M.; Karamanska, R.; Allman, S.; Fairhurst, S.A.; Innocenti, P.; Fairbanks, A.J.; Donohoe, T.J.; Davis, B.G.; Russell, D.A.; Field, R.A. Surface plasmon resonance imaging of glycoarrays identifies novel and unnatural carbohydrate-based ligands for potential ricin sensor development. Chem. Sci. 2011, 2, 1952–1959. [Google Scholar] [CrossRef] [Green Version]
- Koeman, F.A.W.; Meissner, J.W.; van Ritten, H.R.P.; Karmeling, J.P.; Vliegenthart, J.F.G. Synthesis of Structural Elements of the Capsular Polysaccharide of Streptococcus Pneumoniae Type 14. J. Carbohydr. Chem. 1994, 13, 1–25. [Google Scholar] [CrossRef]
- Neilson, T.; Werstiuk, E.S. Oligoribonucleotide Synthesis. II. Preparation of 2′-O-tetrahydropyranyl Derivatives of Adenosine and Cytidine Necessary for Insertion in Stepwise Synthesis. Can. J. Chem. 1971, 49, 493–499. [Google Scholar] [CrossRef]
- Manea, F.; Bindoli, C.; Fallarini, S.; Lombardi, G.; Polito, L.; Lay, L.; Bonomi, R.; Mancin, F.; Scrimin, P. Multivalent, Saccharide-Functionalized Gold Nanoparticles as Fully Synthetic Analogs of Type A Neisseria meningitidis Antigens. Adv. Mat. 2008, 20, 4348–4352. [Google Scholar] [CrossRef]
- Costantino, P.; Norelli, F.; Giannozzi, A.; D′Ascenzi, S.; Bartoloni, A.; Kaur, S.; Tang, D.; Seid, R.; Viti, S.; Paffetti, R.; et al. Size fractionation of bacterial capsular polysaccharides for their use in conjugate vaccines. Vaccine 1999, 17, 1251–1263. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarengo, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals, 2nd ed.; Pergamon Press: Headington Hill Hall, Oxford, UK, 1980. [Google Scholar]
- Kochetkov, N.K.; Nifant′ev, N.E.; Backinowsky, L.V. Synthesis of the capsular polysaccharide of Streptococcus Pneumoniae type 14. Tetrahedron 1987, 43, 3109–3121. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Compound | IC50 (mg/mL) | Maximum Inhibitiona (%) |
|---|---|---|
| SP-14 | 28 × 10−5 ± 1.2 | 100 ± 3 |
| 2 | 1.2 × 10−5 ± 0.5 | 30 ± 5 |
| 3 | 1.4 × 10−5 ± 0.4 | 31 ± 3 |
| 4 | 4.1 × 10−5 ± 0.3 | 31 ± 2 |
| 5 | 5.1 × 10−5 ± 0.4 | 31 ± 3 |
| 6 | 2.8 × 10−5 ± 0.2 | 33 ± 4 |
| 7 | 3.8 × 10−5 ± 0.3 | 34 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gragnani, T.; Cuffaro, D.; Fallarini, S.; Lombardi, G.; D’Andrea, F.; Guazzelli, L. Selectively Charged and Zwitterionic Analogues of the Smallest Immunogenic Structure of Streptococcus Pneumoniae Type 14. Molecules 2019, 24, 3414. https://doi.org/10.3390/molecules24183414
Gragnani T, Cuffaro D, Fallarini S, Lombardi G, D’Andrea F, Guazzelli L. Selectively Charged and Zwitterionic Analogues of the Smallest Immunogenic Structure of Streptococcus Pneumoniae Type 14. Molecules. 2019; 24(18):3414. https://doi.org/10.3390/molecules24183414
Chicago/Turabian StyleGragnani, Tiziana, Doretta Cuffaro, Silvia Fallarini, Grazia Lombardi, Felicia D’Andrea, and Lorenzo Guazzelli. 2019. "Selectively Charged and Zwitterionic Analogues of the Smallest Immunogenic Structure of Streptococcus Pneumoniae Type 14" Molecules 24, no. 18: 3414. https://doi.org/10.3390/molecules24183414
APA StyleGragnani, T., Cuffaro, D., Fallarini, S., Lombardi, G., D’Andrea, F., & Guazzelli, L. (2019). Selectively Charged and Zwitterionic Analogues of the Smallest Immunogenic Structure of Streptococcus Pneumoniae Type 14. Molecules, 24(18), 3414. https://doi.org/10.3390/molecules24183414