Effect of the Chemical Composition of Free-Terpene Hydrocarbons Essential Oils on Antifungal Activity
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of EOs and Their Fractions
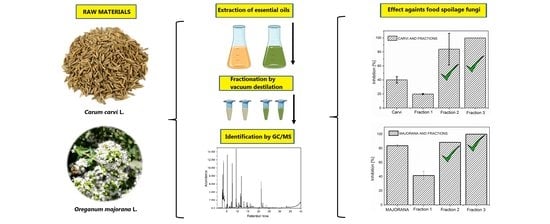
2.2. Antifungal Effect of EOs and Fractions
3. Discussion
4. Materials and Methods
4.1. Solvents and Reagents
4.2. Essential Oils Extraction
4.3. Deterpenation Process
4.4. Identification and Quantification of EOs and Their Fractions
4.5. Isolation of Food Spoilage Fungi
4.6. Identification of the Isolated Fungi
4.7. Antifungal Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Essono, G.; Ayodele, M.; Akoa, A.; Foko, J.; Olembo, S.; Gockowski, J. Aspergillus species on cassava chips in storage in rural areas of southern Cameroon: Their relationship with storage duration, moisture content and processing methods. African J. Microbiol. Res. 2007, 1, 1–8. [Google Scholar]
- Mohamed Abdel-Kader, M.; Samy El-Mougy, N.; Mohamed Lashin, S. Essential oils and trichoderma harzianum as an integrated control measure against faba bean root rot pathogens. J. Plant Prot. Res. 2011, 51, 306–313. [Google Scholar] [CrossRef]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Godevac, D.; Dimkić, I.; Stanković, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crops Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Aminifard, M.H.; Bayat, H. Antifungal Activity of Black Caraway and Anise Essential Oils Against Penicillium digitatum on Blood Orange Fruits. Int. J. Fruit Sci. 2018, 18, 307–319. [Google Scholar] [CrossRef]
- Plavšić, D.V.; Dimić, G.R.; Psodorov, D.B.; Psodorov, D.D.; Šarić, L.Ć.; Čabarkapa, I.S.; Košutić, M.B. Antifungal activity of mentha piperita and carum carvi essential oils. Matica Srp. J. Nat. Sci. 2017, 133, 201–207. [Google Scholar] [CrossRef]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- De Lira Mota, K.S.; De Oliveira Pereira, F.; De Oliveira, W.A.; Lima, I.O.; De Oliveira Lima, E. Antifungal activity of thymus vulgaris l. essential oil and its constituent phytochemicals against Rhizopus oryzae: Interaction with ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef]
- Pawar, V.C.; Thaker, V.S. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 2006, 49, 316–323. [Google Scholar] [CrossRef]
- Ben Salha, G.; Herrera Díaz, R.; Labidi, J.; Abderrabba, M. Deterpenation of Origanum majorana L. essential oil by reduced pressure steam distillation. Ind. Crops Prod. 2017, 109, 116–122. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A 2012, 29, 415–422. [Google Scholar]
- Perini, J.F.; Silvestre, W.P.; Agostini, F.; Toss, D.; Pauletti, G.F. Fractioning of orange (Citrus sinensis L.) essential oil using vacuum fractional distillation. Sep. Sci. Technol. 2017, 52, 1397–1403. [Google Scholar] [CrossRef]
- Brose, D.J.; Chidlaw, M.B.; Friesen, D.T.; LaChapelle, E.D.; Van Eikeren, P. Fractionation of citrus oils using a membrane-based extraction process. Biotechnol. Prog. 1995, 11, 214–220. [Google Scholar] [CrossRef]
- Banchio, E.; Bogino, P.C.; Zygadlo, J.; Giordano, W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008, 36, 766–771. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; Bettaieb, T.; Mougou, A.; Marzouk, B. Essential oils and fatty acids composition of Tunisian, German and Egyptian caraway (Carum carvi L.) seed ecotypes: A comparative study. Ind. Crops Prod. 2013, 41, 312–318. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S. Contamination of common spices in Saudi Arabia markets with potential mycotoxin-producing fungi. Saudi J. Biol. Sci. 2010, 17, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Press: St. Paul, MN, USA, 2013. [Google Scholar]
- Seo, S.-M.; Kim, J.; Lee, S.-G.; Shin, C.-H.; Shin, S.-C.; Park, I.-K. Fumigant Antitermitic Activity of Plant Essential Oils and Components from Ajowan (Trachyspermum ammi), Allspice (Pimenta dioica), Caraway (Carum carvi), Dill (Anethum graveolens), Geranium (Pelargonium graveolens), and Litsea (Litsea cubeba) Oils against Japanese Termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2009, 57, 6596–6602. [Google Scholar] [PubMed]
- Salha, G.B.; Abderrabba, M.; Labidi, J. A status review of terpenes and their separation methods. Rev. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Lago, S.; Rodríguez, H.; Soto, A.; Arce, A. Deterpenation of citrus essential oil by liquid-liquid extraction with 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl) amide ionic liquids. J. Chem. Eng. 2011, 56, 1273–1281. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Mighri, H.; Aouni, M.; Gharsallah, N.; Kadri, A. Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog. 2016, 95, 86–94. [Google Scholar] [CrossRef]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind. Crops Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; Mougou, A.; Marzouk, B. Fatty acid and essential oil composition of three Tunisian caraway (Carum carvi L.) seed ecotypes. J. Sci. Food Agric. 2010, 3, 391–396. [Google Scholar]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Şanlı, A.; Karadoğan, T. Carvone containing essential oils as sprout suppressants in potato (Solanum tuberosum L.) tubers at different storage temperatures. Potato Res. 2019, 3, 345–360. [Google Scholar] [CrossRef]
- Shukla, S.; Pandey, S.S.; Chandra, M.; Pandey, A.; Bharti, N.; Barnawal, D.; Kalra, A. Application of essential oils as a natural and alternate method for inhibiting and inducing the sprouting of potato tubers. Food chem. 2019, 284, 171–179. [Google Scholar] [CrossRef]
- Hromiš, N.; Lazić, V.; Bulut, S.; Popović, S.; Šuput, D.; Markov, S.; Tomović, V. Antimicrobial activity of composite chitosan biofilms with beeswax and caraway essential oil. J. Process. Energy Agric. 2017, 21, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Souza, E.L.D.; Lima, E.D.O.; Freire, K.R.D.L.; Sousa, C.P.D. Inhibitory action of some essential oils and phytochemicals on the growth of various moulds isolated from foods. Braz. Arch. Biol. Technol. 2005, 48, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Moleyar, V.; Narasimham, P. Detoxification of essential oil components (Citral and menthol) by Aspergillus niger and Rhizopus stolonifer. J. Sci. Food Agric. 1987, 39, 239–246. [Google Scholar] [CrossRef]
- Adams, R.P.; Sparkman, O.D. Review of identification of essential oil components by gas chromatography/mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 803–806. [Google Scholar]
- Toma, F.M.; Abdulla, N.Q.F. Isolation and identification of fungi from spices and medicinal plants. Res. J. Environ. Earth Sci. 2013, 5, 131–138. [Google Scholar] [CrossRef]
- Romero, S.M.; Comerio, R.M.; Larumbe, G.; Ritieni, A.; Vaamonde, G.; Fernández Pinto, V. Toxigenic fungi isolated from dried vine fruits in Argentina. Int. J. Food Microbiol. 2005, 104, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D.; Ailsa, D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009. [Google Scholar]
- Solberg, S.O.; Göransson, M.; Petersen, M.A.; Yndgaard, F.; Jeppson, S. Caraway essential oil composition and morphology: The role of location and genotype. Biochem. Syst. Ecol. 2016, 66, 351–357. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Carum carvi L. essential oil and Origanum majorana L. essential oil are available from the authors. |


| Compounds | m/z | RT | CEO | CF1 | CF2 | CF3 | MEO | MF1 | MF2 | MF3 |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |||
| β-terpinen | 93/77/79 | 5.72 | 0.03 ± 0.02 | 0.08 ± 0.03 | - | - | - | - | - | - |
| β-myrcen | 93/69/41 | 5.91 | 0.18 ± 0.02 | 1.54 ± 0.25 | 1.15 ± 0.19 | - | - | - | - | - |
| octanal | 41/43/57 | 6.08 | 0.03 ± 0.01 | 0.55 ± 0.08 | 0.03 ± 0.00 | - | - | - | - | - |
| 2-tujene | 93/9177 | 6.21 | - | - | - | - | 2.24 ± 0.42 | 4.12 ± 1.04 | - | - |
| α-pinene | 93/91/92 | 6.39 | 0.05 ± 0.02 | 0.14 ± 0.02 | 0.05 ± 0.01 | 0.49 ± 0.08 | 0.87 ± 0.16 | 1.61 ± 0.25 | - | - |
| D-limonene | 68/93/67 | 6.55 | 22.22 ± 2.13 | 77.32 ± 4.01 | 18.31 ± 1.96 | 0.07 ± 0.01 | - | - | - | - |
| camphene | 93/121/79 | 6.79 | - | - | - | - | 0.08 ± 0.05 | - | - | - |
| 4(10)-Thujene | 41/94/69 | 7.52 | - | - | - | - | 5.53 ± 0.41 | 11.17 ± 1.03 | - | - |
| β-linalool | 71/93/43 | 7.54 | 0.05 ± 0.01 | 0.08 ± 0.02 | 0.09 ± 0.02 | 0.03 ± 0.00 | - | - | - | - |
| β-pinene | 28/32/93 | 7.60 | - | - | - | - | 0.46 ± 0.07 | 0.90 ± 0.09 | - | - |
| 1R,4R-p-mentha-2,8-dienol | 109/79/94 | 7.97 | 0.20 ± 0.02 | 0.15 ± 0.01 | 0.30 ± 0.05 | 0.15 ± 0.03 | - | - | - | - |
| myrcene | 28/93/41 | 8.05 | - | - | - | - | 1.62 ± 0.09 | 2.77 ± 0.88 | - | - |
| cis-p-Mentha-2,8-dien-1-ol | 134/109/43 | 8.22 | 0.34 ± 0.06 | 0.06 ± 0.00 | 0.44 ± 0.06 | 0.14 ± 0.04 | - | - | - | - |
| limonene oxide | 43/94/67 | 8.29 | 0.07 ± 0.00 | - | 0.39 ± 0.10 | 0.03 ± 0.00 | - | - | - | - |
| α-phellandrene | 93/91/77 | 8.44 | - | - | - | - | 0.58 ± 0.11 | 0.95 ± 0.14 | - | - |
| 2-nonenal | 55/28/41 | 8.64 | 0.04 ± 0.00 | - | 0.07 ± 0.02 | - | - | - | - | - |
| camphenone | 93/108/91 | 8.85 | 0.05 ± 0.00 | - | 0.07 ± 0.01 | 0.04 ± 0.01 | - | - | - | - |
| α-terpinene | 121/93/136 | 8.87 | - | - | - | - | 11.08 ± 0.88 | 21.15 ± 4.52 | 1.48 ± 0.00 | 0.18 ± 0.00 |
| p-cymene | 119/28/134 | 9.08 | - | - | - | - | 2.76 ± 0.54 | 5.20 ± 0.48 | 0.64 ± 0.08 | 0.15 ± 0.04 |
| 2-isopropyl-5-methyl-4-hexanal | 69/84/41 | 9.11 | 0.33 ± 0.09 | - | 0.47 ± 0.04 | 0.10 ± 0.02 | - | - | - | - |
| β-phellandrene | 93/77/91 | 9.23 | - | - | - | - | 4.36 ± 0.25 | 7.81 ± 0.78 | 0.87 ± 0.06 | 0.51 ± 0.07 |
| γ-terpinene | 93/91/136 | 10.27 | - | - | - | - | 15.73 ± 1.20 | 27.53 ± 2.45 | 7.08 ± 1.02 | - |
| carveol | 84/109/134 | 10.33 | 1.55 ± 0.13 | - | 0.65 ± 0.07 | 0.32 ± 0.02 | - | - | - | - |
| trans-sabinene hydrate | 10.47 | - | - | - | - | 1.58 ± 0.24 | 1.30 ± 0.07 | 2.84 ± 0.17 | 0.44 ± 0.07 | |
| 7dihydrocarveol | 93/107/121 | 10.49 | 0.22 ± 0.02 | - | 0.17 ± 0.03 | 0.67 ± 0.09 | - | - | - | - |
| (−)-Carvone | 82/108/93 | 11.07 | 74.25 ± 4.25 | 20.18 ± 1.63 | 78.21 ± 3.69 | 97.15 ± 5.97 | - | - | - | - |
| α-Terpinolene | 11.20 | - | - | - | - | 3.82 ± 0.56 | - | 2.03 ± 1.11 | 3.43 ± 0.89 | |
| cis-sabinene hydrate | 11.53 | - | - | - | - | 4.52 ± 0.76 | 1.88 ± 0.30 | 10.00 ± 1.56 | 5.79 ± 0.78 | |
| linalool | 71/28/93 | 11.60 | - | - | - | - | 1.16 ± 0.09 | 0.39 ± 0.01 | 1.82 ± 0.77 | 0.67 ± 0.22 |
| perilla aldéhyde | 135/77/93 | 11.82 | 0.21 ± 0.02 | - | 0.22 ± 0.10 | 0.67 ± 0.14 | - | - | - | - |
| trans-p-menth-2-enol | 12.30 | - | - | - | - | 2.05 ± 0.02 | - | 3.13 ± 0.92 | 1.90 ± 0.41 | |
| cis-β-Terpineol | 28/43/93 | 12.92 | - | - | - | - | 1.36 ± 0.33 | 0.52 ± 0.09 | 1.86 ± 0.20 | 2.76 ± 0.38 |
| endo-borneol | 13.85 | - | - | - | - | 0.23 ± 0.04 | - | - | 0.46 ± 0.05 | |
| thymol | 135/150/91 | 14.23 | - | - | - | - | - | - | - | 0.30 ± 0.06 |
| terpine-4-ol | 71/111/93 | 14.35 | - | - | - | - | 27.32 ± 2.21 | 7.30 ± 1.22 | 54.39 ± 3.25 | 48.60 ± 4.87 |
| β-fenchyl alcohol | 14.40 | - | - | - | - | - | - | 9.63 ± 1.54 | 23.84 ± 2.39 | |
| p-cymen-8-ol | 14.50 | - | - | - | - | 0.08 ± 0.00 | - | - | - | |
| trans-piperitol | 14.81 | - | - | - | - | 0.64 ± 0.04 | - | 0.57 ± 0.07 | 2.61 ± 0.37 | |
| cis-piperitol | 28/32/18 | 15.17 | - | - | - | - | 0.73 ± 0.09 | - | 0.82 ± 0.04 | 1.79 ± 0.11 |
| carvacrol | 135/150/91 | 17.89 | - | - | - | - | 0.21 ± 0.00 | - | - | - |
| caryophyllene | 133/93/91 | 20.78 | - | - | - | - | 0.83 ± 0.07 | - | 0.80 ± 0.04 | 2.70 ± 0.44 |
| aromandendrene | 21.23 | - | - | - | - | 0.05 ± 0.00 | - | - | 0.33 ± 0.10 | |
| α-humulene | 21.55 | - | - | - | - | 0.05 ± 0.01 | - | - | - | |
| (+)-Bicyclogermacrene | 28/32/18 | 22.50 | - | - | - | - | 0.65 ± 0.20 | - | - | 1.68 ± 0.17 |
| spathulenol | 43/41/205 | 24.18 | - | - | - | - | 0.24 ± 0.04 | - | - | 0.20 ± 0.02 |
| caryophylleneoxide | 79/43/93 | 24.30 | - | - | - | - | 0.15 ± 0.00 | - | - | - |
| linalylacetate | 93/28/43 | 16.60 | - | - | - | - | 0.56 ± 0.10 | - | 0.65 ± 0.09 | 1.23 ± 0.22 |
| Isobornyl acetate | 28/32/95 | 17.41 | - | - | - | - | 0.26 ± 0.02 | - | 0.41 ± 0.03 | |
| α-terpenylpropionate | 17.61 | - | - | - | - | - | - | 1.39 ± 0.21 | - | |
| 4-Terpinenyl acetate | 93/121/136 | 17.81 | - | - | - | - | 1.03 ± 0.18 | - | - | - |
| Mass (g) | 20.12 | 4.1 | 5.62 | 10.40 | 22.00 | 8.88 | 10.13 | 0.99 | ||
| Boiling temperature (at 10 mmHg) | - | 65–75 | 80–90 | 110 | - | 52–54 | 70–72 | 84–86 | ||
| Total terpenes hydrocarbons (%) | 22.48 | 79.06 | 19.47 | 0.56 | 50.70 | 87.92 | 12.90 | 8.98 | ||
| Total oxygenated terpenes (%) | 77.43 | 21.62 | 79.21 | 98.56 | 47.36 | 12.05 | 85.06 | 88.86 | ||
| Samples | CEO | CF1 | CF2 | CF3 | MEO | MF1 | MF2 | MF3 |
|---|---|---|---|---|---|---|---|---|
| % FGI R. oryzae | 83.68 ± 1.75 | 41.51 * ± 5.95 | 88.49 ± 0.12 | 100.00 ± 0.00 | 85.84 ± 1.84 | 16.41 * ± 1.04 | 99.84 ± 0.22 | 97.02 ± 4.21 |
| % FGI R. stolonifier | 84.29 ± 2.56 | 43.69 * ± 1.38 | 87.93 ± 1.96 | 100.00 ± 0.00 | 53.40 * ± 0.58 | 12.85 * ± 3.58 | 100.00 ± 0.00 | 98.71 ± 1.81 |
| % FGI A. penicillioides | 40.15 ± 4.68 | 19.92 ± 0.85 | 84.01 ± 12.62 | 100.00 ± 0.00 | 29.95 ± 3.79 | 11.53 ± 2.17 | 100.00 ± 0.00 | 98.29 ± 2.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Salha, G.; Herrera Díaz, R.; Lengliz, O.; Abderrabba, M.; Labidi, J. Effect of the Chemical Composition of Free-Terpene Hydrocarbons Essential Oils on Antifungal Activity. Molecules 2019, 24, 3532. https://doi.org/10.3390/molecules24193532
Ben Salha G, Herrera Díaz R, Lengliz O, Abderrabba M, Labidi J. Effect of the Chemical Composition of Free-Terpene Hydrocarbons Essential Oils on Antifungal Activity. Molecules. 2019; 24(19):3532. https://doi.org/10.3390/molecules24193532
Chicago/Turabian StyleBen Salha, Ghada, René Herrera Díaz, Olfa Lengliz, Manef Abderrabba, and Jalel Labidi. 2019. "Effect of the Chemical Composition of Free-Terpene Hydrocarbons Essential Oils on Antifungal Activity" Molecules 24, no. 19: 3532. https://doi.org/10.3390/molecules24193532
APA StyleBen Salha, G., Herrera Díaz, R., Lengliz, O., Abderrabba, M., & Labidi, J. (2019). Effect of the Chemical Composition of Free-Terpene Hydrocarbons Essential Oils on Antifungal Activity. Molecules, 24(19), 3532. https://doi.org/10.3390/molecules24193532