Identification and Migration Studies of Photolytic Decomposition Products of UV-Photoinitiators in Food Packaging
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Photolytic Decomposition of PI’s
2.2. Evaluation of the Occurrence and Migration Level of PI’s and Photolytic Decomposition Products
3. Discussion
4. Materials and Methods
4.1. Food Packaging Samples
4.2. Reagents, Reference Standards, and Materials
4.3. UV-Irradiation of PI Standards
4.4. Migration Testing
4.5. Sample Preparation of Migration Test Extracts for GC-MS Analysis
4.6. GC-MS Analysis Methodology
4.7. ESI-MS Analysis Methodology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersson, E. Starch and Hemicellulose as Barrier Materials in Food Packaging. Master’s Thesis, Karlstad University, Karlstad, Sweden, 2015. [Google Scholar]
- Vesley, G.F. Mechanism of the photodecomposition of initiators. J. Radiat. Curing. 1986, 13, 4–10. [Google Scholar]
- Lago, M.A.; Rodriguez-Bernaldo de Quiros, A.; Sendon, R.; Bustos, J.; Nieto, M.T.; Paserio, P. Photoinitiators: A food safety review. Food Addit. Contam. Part A: Chem. Anal. Control Expo. Risk Assess. 2015, 32, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Bosnea, L. Migration of substances from food packaging materials to foods. Crit. Rev. Food Sci. Nutr. 2010, 44, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Kirschmayr, R.; Berner, G.; Huelser, R.; Rist, G. Nonyellowing photoinitiators. Farbe Lack. 1982, 88, 910–914. [Google Scholar]
- Buck, K.; Bussey, R. Product safety and consumer acceptance in food-packaging applications. Cereal Foods World 2002, 47, 425–428. [Google Scholar]
- Aparicio, J.L.; Elizalde, M. Migration of photoinitiators in food packaging: A review. Packag. Technol. Sci. 2015, 28, 181–203. [Google Scholar] [CrossRef]
- EuPIA Suitability List of Photo-Initiators for Low Migration UV Printing Inks and Varnishes. Available online: http://www.eupia.org/fileadmin/user_upload/Printing_ink_for_food_packaging/130219_corr3_EuPIA_Suitability_List_of_Photoinitiators_for_Low_migration_UV_Printing_Inks_and_Varnishes.pdf (accessed on 6 July 2018).
- Nestle Guidance Note on Packaging Inks. Available online: http://www.argus-analysen.de/assets/plugindata/poola/nestle-guidance-note-on-packaging-inks-2016-08.pdf (accessed on 21 July 2018).
- Ordinance of the FDA on Articles and Materials, Annex 6. Lists of Permitted Substances for the Manufacture of Packaging Inks, Subject to the Requirements Set out Herein. Available online: https://www.blv.admin.ch/dam/blv/en/dokumente/gebrauchsgegenstaende/rechts-und-vollzugsgrundlagen/anhang-6-sr-bedarfsgegenstaende.pdf.download.pdf/130401+Annex+6_en.pdf (accessed on 24 September 2018).
- Proposition 65: Safe Drinking Water and Toxic Enforcement Act. Available online: https://oehha.ca.gov/proposition-65 (accessed on 13 October 2018).
- GRAS Notice Inventory. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm (accessed on 27 September 2018).
- Union Guidance on Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food as Regards Information in the Supply Chain. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_fcm_plastic-guidance_201110_reg_en.pdf (accessed on 21 August 2018).
- Zhang, N.; Kenion, G.; Bankmann, D.; Mezouari, S.; Hartman, T. Migration studies and chemical characterization of low molecular weight cyclic polyester oligomers from food packaging lamination adhesives. Packag. Technol. Sci. 2018, 1–16. [Google Scholar] [CrossRef]
- Green, W.A. Photoproducts and the yellowing of coatings. In Industrial Photoinitiators: A Technical Guide; CRC Press: Boca Raton, FL, USA, 2010; pp. 139–147. [Google Scholar]
- Lago, M.A.; Ackerman, L.K. Identification of print-related contaminants in food packaging. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Green, W.A. A little chemistry. In Industrial Photoinitiators: A Technical Guide; CRC Press: Boca Raton, FL, USA, 2010; pp. 18–22. [Google Scholar]
- Ligon, S.C.; Husar, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef]
- Arsu, N.; Davidson, R.S.; Holman, R. Factors affecting the photoyellowing which occurs during the photoinitiated polymerization of acrylates. J. Photochem. Photobio. A Chem. 1995, 87, 169–175. [Google Scholar] [CrossRef]
- Schug, K.; McNair, H.M. Adduct formation in electrospray ionization. Part 1: Common acidic pharmaceuticals. J. Sep. Sci. 2002, 25, 759–766. [Google Scholar] [CrossRef]
- Smith, L.E.; Chang, S.S.; McCrackin, F.L.; Senich, G.A.; Wang, F.W. Experimental measurement of migration by extraction. In Models for the Migrations ofLow Molecular Weight Additives in Polyolefins; Polymer Science and Standards Division, National Bureau of Standards, US Department of Commerce: Washington, DC, USA, 1981; pp. 13–18. [Google Scholar]
- Preparation of Premarket Submissions for Food Contact Substances (Chemistry Recommendations). Available online: https://www.fda.gov/RegulatoryInformation/Guidances/ucm081825.htm#iva2 (accessed on 21 July 2018).
- Misev, L. Selection criteria for high performance photoinitiators in pigmented systems. In Proceedings of Radtech Asia; Radtech Asia: Beijing, China, 1991. [Google Scholar]
- Kung, R. Polymeric photoinitiators: UV inks and coatings for food packaging. Radtech Rep. 2011, 43–48. [Google Scholar]
- Ludwig, P.E.; Ossenbach, A.J. Photoinitiators: Challenges in food packaging applications. UV + EB Tech. 2016, 4. [Google Scholar]
- General Provisions Applicable to Indirect Food Additives. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=174.5 (accessed on 29 September 2019).
- Threshold of Regulation for Substances Used in Food-Contact Articles. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=170.39 (accessed on 27 July 2018).
- Food Additive Status List. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on 31 July 2019).
- Food Additive Regulations; Synthetic Flavoring Agents and Adjuvants. Available online: https://www.regulations.gov/document?D=FDA-2015-F-4317-0058 (accessed on 31 July 2019).
- Food Contact Materials- Regulation (EC) 1935/2004. Available online: http://www.europarl.europa.eu/RegData/etudes/STUD/2016/581411/EPRS_STU(2016)581411_EN.pdf (accessed on 9 October 2018).
Sample Availability: The complete set of raw data including mass spectra of compounds identified is available from the authors upon request. |


| Reference Number | CAS Number | IUPAC Nomenclature | Structure |
|---|---|---|---|
| 1 | 7473-98-5 | 2-hydroxy-2-methyl-1-phenylpropanone |  |
| 2 | 947-19-3 | 1-hydroxycyclohexyl-1-phenylmethanone | 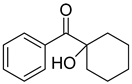 |
| 3 | 106797-53-9 | 1-[4-(2-hydroxyethoxyl)-phenyl]-2-hydroxy-2-methyl propanone |  |
| 4 | 163702-01-1 | oligo{2-hydroxy-2-methyl-1-[4-(1-methylvinyl)phenyl] propanone} |  |
| 5 | 474510-57-1 | 2-hydroxy-1-{4-[4-(2-hydroxy-2-methylpropionyl)benzyl] phenyl)-2-methylpropan-1-one | 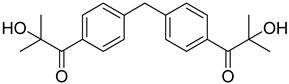 |
| 6 | 163702-01-0 | 2-hydroxy-1-{1-[4-(2-hydroxy-2-methylpropionyl) phenyl]1,3,3-trimethylindan-5-yl-}2-methylpropan-1-one | 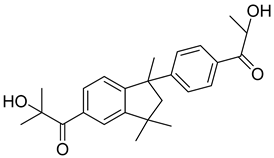 |
| 7 | 71868-10-5 | 2-methyl-1-[4-(methylthio) phenyl]-2-morpholinopropan-1-one |  |
| 8 | 119313-12-1 | 2-benzyl-2-(dimethylamino)-4’-morpholinobutyrophenone | 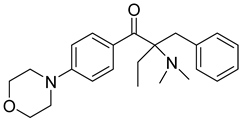 |
| 9 | 119344-86-4 | 2-dimethylamino-2-(4-methylbenzyl)-1-(4-morpholin-4-yl-phenyl) butan-1-one | 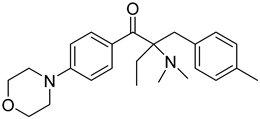 |
| 10 | 24650-42-8 | 2,2-dimethoxy-2-phenylacetophenone | 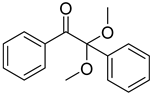 |
| 11 | 75980-60-8 | 2,4,6-trimethylbenzoyl-diphenylphosphine oxide |  |
| 12 | 84434-11-7 | ethyl 2,4,6-trimethylbenzoylphenyl phosphinate | 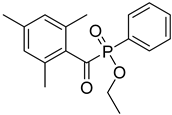 |
| 13 | 162881-26-7 | bis(2,4,6-trimethylbenoyl)phenylphosphine oxide | 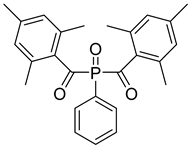 |
| 14 | 119-61-9 | benzophenone | 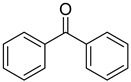 |
| 15 | 134-84-9 | 4-methylbenzophenone | 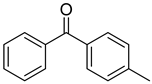 |
| 16 | 954-16-5 | 2,4,6-trimethylbenzophenone | 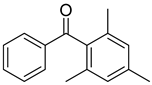 |
| 17 | 2128-93-0 | 4-phenylbenzophenone | 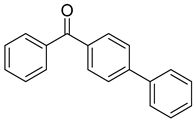 |
| 18 | 83846-85-9 | 4-(4-methylphenylthio) benzophenone | 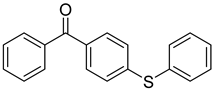 |
| 19 | 606-28-0 | methyl 2-benzoylbenzoate | 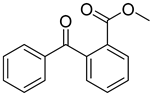 |
| 20 | 83846-86-0 | 2-isopropylthioxanthone | 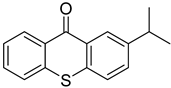 |
| 21 | 82799-44-8 | 2,4-diethylthioxanthone | 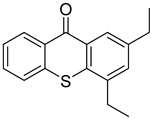 |
| 22 | 90-93-7 | 4,4’-bis(diethylamino) benzophenone | 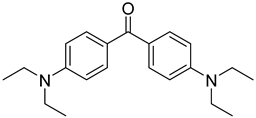 |
| 23 | 21245-02-3 | 2-ethylhexyl 4-dimethylaminobenzoate |  |
| 24 | 10287-53-3 | ethyl 4-dimethylaminobenzoate |  |
| Photolytic Decomposition Product | CAS Number | MW | Parent PI | Peak Area % 1 | RI 2 |
|---|---|---|---|---|---|
| N-methylpropanamide | 1187-58-2 | 87 | 8 | 0.019 | 1026 |
| 9 | 0.010 | ||||
| cyclohexanone | 108-94-1 | 98 | 2 | 0.458 | 898 |
| cyclohexanol | 108-93-0 | 100 | 2 | 0.004 | 894 |
| N,N-dimethylpropanamide | 758-96-3 | 101 | 8 | 0.056 | 973 |
| 9 | 0.029 | ||||
| benzaldehyde | 100-52-7 | 106 | 1 | 0.186 | 966 |
| 2 | 0.264 | ||||
| 4 | 0.006 | ||||
| 8 | 0.033 | ||||
| 10 | 0.105 | ||||
| p-xylene | 106-42-3 | 106 | 9 | 0.159 | 872 |
| phenylphosphine | 638-21-1 | 110 | 13 | 0.069 | 917 |
| 2-hydroxycyclohexanone | 533-60-8 | 114 | 2 | 0.069 | 1003 |
| 2-methoxythiazole | 14542-13-3 | 115 | 7 | 0.063 | 1188 |
| N,N-dimethyl-3-methoxypropylamine | 20650-07-1 | 117 | 9 | 0.023 | 1592 |
| acetophenone | 98-86-2 | 120 | 1 | 0.123 | 1080 |
| 1,3,5-trimethylbenzene | 108-67-8 | 120 | 11 | 0.319 | 971 |
| 12 | 4.832 | ||||
| 13 | 4.526 | ||||
| 15, 16 6 | 0.002 | ||||
| 1-phenylethenol 4 | 4383-15-7 | 120 | 3 | 0.013 | 1112 |
| N,N-dimethylaniline | 121-69-7 | 121 | 23 | 0.002 | 1098 |
| benzoic acid | 65-85-0 | 122 | 1 | 0.732 | 1126 |
| 2 | 0.107 | ||||
| 10 | 0.269 | ||||
| 10 | 0.012 | ||||
| 4-hydroxybenzaldehyde | 123-08-0 | 122 | 3 | 0.027 | 1674 |
| 4-methylbenzenethiol | 106-45-6 | 124 | 18 | 0.002 | 1075 |
| methyl phenyl sulfide | 100-68-5 | 124 | 7 | 0.014 | 1092 |
| 4-acetylmorpholine | 1696-20-4 | 129 | 7 | 0.038 | 1259 |
| (E)-2-phenyl-2-butene | 768-00-3 | 132 | 8 | 0.032 | 1063 |
| (E)-1-phenyl-1-butene | 1005-64-7 | 132 | 8 | 0.007 | 1132 |
| 1,3-diethylbenzene | 141-93-5 | 134 | 21 | 0.003 | 1054 |
| benzothiazole | 95-16-9 | 135 | 7 | 0.003 | 1243 |
| methyl benzoate | 93-58-3 | 136 | 1 | 0.013 | 1103 |
| 10 | 1.248 | ||||
| 2,4,6-trimethylphenol | 527-60-6 | 136 | 11 | 0.015 | 1216 |
| 12 | 0.399 | ||||
| 13 | 0.128 | ||||
| 15, 16 6 | 0.002 | ||||
| α,α-dimethylbenzyl alcohol | 617-94-7 | 136 | 1 | 0.249 | 1282 |
| 4-aminobenzoic acid | 150-13-0 | 137 | 24 | 0.001 | 1594 |
| 4-methoxybenzenethiol | 696-63-9 | 140 | 7 | 0.027 | 1405 |
| 2-methyl-1-phenyl-1-butene | 56253-64-6 | 146 | 9 | 0.034 | 1170 |
| 3-phenyl-2-propenamide | 621-79-4 | 147 | 9 | 0.001 | 1178 |
| 6-methyl-1,2,3,4-tetrahydroquinoline | 91-61-2 | 147 | 8 | 0.008 | 1382 |
| benzoyl methyl ketone | 579-07-7 | 148 | 1 | 0.021 | 1178 |
| 1-phenyl-2-butanone | 1007-32-5 | 148 | 8 | 1.050 | 1230 |
| 4-isopropylbenzaldehyde | 123-03-2 | 148 | 4 | 0.001 | 1252 |
| 2,4,6-trimethylbenzaldehyde | 487-68-3 | 148 | 11 | 0.113 | 1314 |
| 12 | 10.05 | ||||
| 13 | 0.996 | ||||
| N,N-diethylaniline | 91-66-7 | 149 | 22 | 0.004 | 1232 |
| N,N-dimethylphenethylamine | 1126-71-2 | 149 | 8 | 0.036 | 1500 |
| methyl phenyl carbonate | 13509-27-8 | 152 | 10 | 0.024 | 1155 |
| benzaldehyde dimethyl acetal | 1122-88-8 | 152 | 10 | 0.039 | 1118 |
| 4-(methylthio)benzaldehyde | 3446-89-7 | 152 | 7 | 1.440 | 1442 |
| biphenyl | 92-52-4 | 154 | 14 | 0.010 | 1393 |
| 17 | 0.006 | ||||
| 3-methyl-1-phenyl-3-buten-2-one | 55956-30-4 | 160 | 8 | 0.004 | 1318 |
| 1-phenyl-1-penten-3-one | 3152-68-9 | 160 | 8 | 0.011 | 1322 |
| 4′-isopropenylacetophenone | 5359-04-6 | 160 | 4 | 0.005 | 1334 |
| 2-methyl-1-phenyl-1-butanone | 938-87-4 | 162 | 8 | 0.010 | 1286 |
| 2-hydroxy-2-methylpropiophenone | 7473-98-5 | 162 | 6 | 0.057 | 1295 |
| 4-phenylmorpholine | 92-53-5 | 163 | 8 | 0.094 | 1439 |
| 9 | 0.146 | ||||
| N,N-dimethylbenzeneacetamide | 18925-69-4 | 163 | 8 | 0.019 | 1498 |
| 2,4,6-trimethylbenzoic acid | 480-63-7 | 164 | 11 | 5.351 | 1467 |
| 12 | 1.390 | ||||
| 13 | 44.52 | ||||
| 2-(2,4,6-trimethylphenyl)ethanol 4 | 6950-92-1 | 164 | 13 | 0.029 | 1246 |
| 2-ethoxy-1,3,5-trimethylbenzene 4 | 61248-63-3 | 164 | 12 | 0.176 | 1249 |
| 4-(dimethylamino)benzoic acid | 619-84-1 | 165 | 23 | 0.002 | 2131 |
| 4-(methylthio)acetophenone | 1778-09-2 | 166 | 7 | 0.025 | 1547 |
| 3-(4-methoxyphenyl)-1-propanol | 5406-18-8 | 166 | 3 | 0.567 | 1604 |
| 3H-1,2-benzodithiol-3-one | 1677-27-6 | 167 | 20 | 0.009 | 1568 |
| 4-(methylthio)benzoic acid | 13205-48-9 | 168 | 7 | 0.353 | 1620 |
| diphenylmethane | 101-81-5 | 168 | 14 | 0.011 | 1448 |
| diphenyl ether | 101-84-8 | 170 | 2 | 0.005 | 1411 |
| 10 | 0.001 | ||||
| 14 | 0.005 | ||||
| 15 | 0.001 | ||||
| 15, 16 6 | 0.001 | ||||
| ethyl phenylphosphinate 4 | 2511-09-3 | 170 | 12 | 64.82 | 1418 |
| cyclopentyl phenyl ketone | 5422-88-8 | 174 | 2 | 0.016 | 1574 |
| 2-methyl-1-phenyl-1,3-butanedione | 6668-24-2 | 176 | 9 | 0.001 | 1340 |
| methyl 2,4,6-trimethylbenzoate | 2282-84-0 | 178 | 11 | 0.001 | 1355 |
| 12 | 0.868 | ||||
| 13 | 0.010 | ||||
| 4-morpholinylaniline | 2524-67-6 | 178 | 9 | 0.001 | 2032 |
| methyl 4-(dimethylamino)benzoate | 1202-25-1 | 179 | 23 | 0.001 | 1651 |
| 1-methoxyethyl benzoate | 51835-44-0 | 180 | 15 | 0.002 | 1295 |
| triethyl phosphate | 78-40-0 | 182 | 12 | 0.271 | 1160 |
| benzophenone | 119-61-9 | 182 | 18 | 0.001 | 1644 |
| 2,2′-dimethylbiphenyl | 605-39-0 | 182 | 15, 16 6 | 0.004 | 1524 |
| 1,2-diphenylethane | 103-29-7 | 182 | 8 | 0.498 | 1539 |
| 2-(3-methylphenoxy)pyridine 4 | 1793003-62-9 | 185 | 9 | 0.001 | 1694 |
| diphenylphosphine | 829-85-6 | 186 | 12 | 0.035 | 1585 |
| 2-phenoxyphenol | 2417-10-9 | 186 | 2 | 0.010 | 1681 |
| cyclohexyl phenyl ketone | 712-50-5 | 188 | 2 | 0.006 | 1620 |
| 4-morpholinobenzaldehyde | 1204-86-0 | 191 | 8 | 1.093 | 1910 |
| ethyl 2,4,6-trimethylbenzoate | 1754-55-8 | 192 | 11 | 0.018 | 1829 |
| 4-(diethylamino)benzoic acid | 5429-28-7 | 193 | 22 | 0.006 | 1854 |
| 15, 16 6 | 0.009 | ||||
| 1-(methylthio)-4-(2-methyl-3-hydroxy-2-propene)-yl-benzene 4 | N/A | 194 | 7 | 0.350 | 1675 |
| 2,2′-dimethyldiphenylmethane 4 | 1634-74-8 | 196 | 15, 16 6 | 0.007 | 1632 |
| p-morpholinoacetophenone | 39910-98-0 | 205 | 9 | 0.020 | 1990 |
| methyl 4-(diethylamino) benzoate 4 | 91563-80-3 | 207 | 22 | 0.008 | 1833 |
| 4′-(2-hydroxyethoxy)-2-methylpropiophenone 4 | N/A | 208 | 3 | 0.692 | 1858 |
| diphenylethanedione | 134-81-6 | 210 | 1 | 0.488 | 1857 |
| 2 | 0.121 | ||||
| 10 | 0.297 | ||||
| 1,2-di(4-methylphenyl)ethane | 538-39-6 | 210 | 9 | 0.897 | 1800 |
| thioxanthone | 492-22-8 | 212 | 20 | 0.010 | 2129 |
| diethyl phenylphosphonate | 1754-49-0 | 214 | 12 | 16.59 | 1514 |
| methyldiphenylphosphine oxide | 2129-89-7 | 216 | 11 | 0.019 | 2028 |
| diphenylphosphinic acid | 1707-03-5 | 218 | 13 | 0.007 | 1897 |
| 4-hydroxychalcone | 2657-25-2 | 224 | 20 | 0.001 | 1857 |
| methyl diphenylphosphinate | 1706-90-7 | 232 | 12 | 0.122 | 1951 |
| 2-ethylhexyl benzoate | 5444-75-7 | 234 | 23 | 0.001 | 1746 |
| diphenylpropanetrione | 643-75-4 | 238 | 15, 16 6 | 0.365 | 2041 |
| 3-methyl-1,8,9-anthracenetriol | 491-59-8 | 240 | 21 | 0.028 | 2361 |
| bis(4-methylphenyl)disulfide | 103-19-5 | 246 | 18 | 0.102 | 2082 |
| ethyl diphenylphosphinate4 | 1733-55-7 | 246 | 11 | 0.200 | 1988 |
| 12 | 0.445 | ||||
| 2,2-dimethyl-1,3-diphenyl-1,3-propanedione | 41169-42-0 | 252 | 4 | 0.001 | 2021 |
| 2-ethylhexyl-2-ethylhexanoate | 7425-14-1 | 256 | 23 | 0.008 | 1608 |
| N,N’-diethyl-N,N’-diphenylurea | 85-98-3 | 268 | 22 | 0.006 | 1914 |
| dimer of 2,4,6-trimethylphenol 3 | N/A | 272 | 13 | 0.039 | 2192 |
| N-hydroxy-2-methoxy-N-methylbenzene Carbothioamide 4 | 95096-17-6 | 273 | 7 | 0.111 | 1878 |
| N-benzyl-N-hydroxy-2-methoxy benzenecarbothioamide | 93979-07-8 | 273 | 7 | 0.010 | 2481 |
| N-ethyl-2-(ethylphenylamino)-N-phenylacetamide 4 | N/A | 282 | 22 | 0.002 | 1978 |
| 2,4,6-trimethylphenyl benzoate 4 | N/A | 282 | 15, 16 6 | 0.007 | 1632 |
| O-dibenzoylbenzene | 1159-86-0 | 286 | 19 | 0.014 | 2516 |
| dimer of 2,4,6-trimethylbenzaldehyde 3 | N/A | 296 | 13 | 5.168 | 2063 |
| 4-methyl-2-(1-phenyl ethyl)phenyl benzoate | 18062-71-0 | 316 | 1 | 0.077 | 2617 |
| dimer of 2,4,6-trimethylacetophenone 3 | N/A | 324 | 13 | 0.152 | 2258 |
| dimer of PI 1 3 | N/A | 328 | 1 | 1.531 | 2219 |
| 1-[(4-diethylamino)phenyl]-1-[(4-diethylamino-3-methylamino)phenyl]methanone 4 | N/A | 353 | 22 | 0.026 | 3231 |
| 1,1,2,2-tetraphenyl-1,2-ethanediol | 464-72-2 | 366 | 14 | 0.012 | 2189 |
| dimer of PI 4 5 | 163702-01-0 | 408 | 4 | 98.39 | 3020 |
| dimer of PI 10 3 | N/A | 512 | 10 | 0.395 | 2941 |
| dimer of PI 11 3 | N/A | 696 | 11 | 8.898 | 3212 |
| trimer of PI 10 3 | N/A | 768 | 10 | 0.036 | 3272 |
| Name | CAS Number | Frequency of Occurrence 1 | 10% Ethanol 2 Concentration Range (ng/cm2) | 95% Ethanol 3 Concentration Range (ng/cm2) |
|---|---|---|---|---|
| benzophenone (PI 14) | 119-61-9 | 88 | 0.24–62.84 c | 1.38–66.30 c |
| 0.04–948.37 e | 0.15–266.20 e | |||
| 6.61–9.64 m | ||||
| 2-hydroxy-2-methyl-1-phenylpropanone (PI 1) | 7473-98-5 | 139 | 0.6 a | 1.22–17.98 a |
| 0.01–499.55 c | 19.42–737.87 c | |||
| 0.25–361.17 e | 0.53–1556.64 e | |||
| 65.65 h | 81.08 h | |||
| 1-hydroxycyclohexyl-1-phenylmethanone (PI 2) | 947-19-3 | 73 | 1426.48c | 49.09–2238.76 c |
| 0.27–1169.02 e | 1.55–1163.71 e | |||
| 753.25 h | 1292.84 h | |||
| 2,2-dimethoxy-2-phenylacetophenone (PI 10) | 24650-42-8 | 49 | 9.27–673.67 c | 14.55–2141.64 c |
| 0.05–109.75 e | 0.55–454.63 e | |||
| 80.22 h | 344.30 h | |||
| 0.33–0.99 m | 0.85–3.97 m | |||
| methyl 2-benzoylbenzoate (PI 19) | 606-28-0 | 56 | 4.29–1854.87c | 3376.41 c |
| 0.10–786.09e | 3.33–1072.49 e | |||
| 520.18 h | 471.65 h | |||
| 2-methyl-1-[4-(methylthio) phenyl]-2-morpholinopropan-1-one (PI 7) | 71868-10-5 | 83 | 3.89–2090.98 c | 2.95–4429.78 c |
| 0.28–365.98 e | 0.93–1209.68 e | |||
| 156.68 h | 671.34 h | |||
| 2-benzyl-2-(dimethylamino)-4′-morpholinobutyrophenone (PI 8) | 119313-12-1 | 3 | 6.79 e | 39.02–51.98 e |
| 2,4,6-trimethylbenzoyl diphenylphosphine oxide (PI 11) | 75980-60-8 | 21 | 236.27–702.12 c | 40.64–1603.21 c |
| 6.40–121.20 e | 79.17–9122.66 e | |||
| ethyl 2,4,6-trimethylbenzoyl phenylphosphinate (PI 12) | 84434-11-7 | 2 | 435.63–904.32 e | |
| 4-phenylbenzophenone (PI 13) | 2128-93-0 | 16 | 14.12–16.73 c | 6.29–155.79c |
| 1.83–51.71 e | 0.73–13.07e | |||
| 26.27 h | 41.37h | |||
| 4-methylbenzophenone (PI 15) | 134-84-9 | 7 | 11.93–87.54 e | |
| 2-isopropylthioxanthone (PI 20) | 83846-86-0 | 20 | 0.32 c | 7.97 c |
| 1.88–178.97 e | 4.68–3221.92 e | |||
| 166.04 h | 1550.84 h | |||
| 2,4-diethylthioxanthone (PI 21) | 82799-44-8 | 25 | 11.21–11.34 c | 896.93–2762.95 c |
| 1.03–2.45 e | 5.27–2227.32 e | |||
| ethyl 4-dimethyl aminobenzoate (PI 24) | 10287-53-3 | 70 | 0.29–892.28 c | 22.647–1637.24 c |
| 0.37–439.49 e | 0.50–1701.19 e | |||
| 153.09–1746.04 h | 255.19–389.86 h | |||
| 2-ethylhexyl- 4-dimethylaminobenzoate (PI 23) | 21245-02-3 | 6 | 59.62–1019.10 e | |
| 4,4′-bis(diethylamino) benzophenone (PI 22) | 90-93-7 | 6 | 0.86–11.48 e | 0.99–3832.71 e |
| {2-hydroxy-2-methyl-1-[4-(1-methylvinyl)phenyl] propanone} (PI 4) | 163702-01-1 | 2 | 11.99a | 6.96 a |
| benzaldehyde | 100-52-7 | 78 | 0.17–33.06 c | 1.61–62.01 c |
| 0.03–111.20 e | 0.44–68.86 e | |||
| 11.20 h | 12.96 h | |||
| acetophenone | 98-86-2 | 38 | 16.49 c | 29.13 c |
| 0.22–10.95 e | 0.24–24.76 e | |||
| 3.75 h | 2.23 h | |||
| methyl benzoate | 93-58-3 | 33 | 0.19–450.52 c | 1.61–548.03 c |
| 0.10–36.05 e | 0.18–78.87 e | |||
| 2.11 h | 8.59 h | |||
| 1.47–2.89 m | ||||
| 2,4,6-trimethylbenzaldehyde | 487-68-3 | 130 | 24.57 a | |
| 0.03–1938.56 c | 0.84–26.96 c | |||
| 0.24–22.22 e | 0.26–64.59 e | |||
| 8.00h | 8.12 h | |||
| 4-methylthiobenzaldehyde | 3446-89-7 | 81 | 0.22–177.49c | 0.69–267.70 c |
| 0.40–24.80e | 0.39–237.19 e | |||
| 28.39h | ||||
| 1-phenyl-2-butanone | 1007-32-5 | 83 | 1.14a | 2.70–50.90 a |
| 0.07–0.81c | 10.55–12.69 c | |||
| 0.06–440.83 e | 0.60–423.52 e | |||
| 0.92–1.87 g | ||||
| 6.33–13.69 h | 0.31–4.70 h | |||
| α,α-dimethylbenzyl alcohol | 617-94-7 | 68 | 7.87–25.09 c | 0.63–23.44 c |
| 0.05–18.68 e | 0.28–258.54 e | |||
| 1.44 h | 1.74 h | |||
| 2,4,6-trimethylbenzoic acid | 480-63-7 | 44 | 17.14–52.12 c | 7.32–92.44 c |
| 0.10–14.38 e | 0.35–156.39 e | |||
| 0.29 h | ||||
| benzothiazole | 95-16-9 | 12 | 1.29 c | |
| 0.18–5.41 e | 0.19–1.32 e | |||
| biphenyl | 92-52-4 | 6 | 0.20–4.16 e | 12.57 e |
| cyclohexanone | 108-94-1 | 15 | 1.51–52.71 e | 2.34–27.56 e |
| p-diisopropenylbenzene | 1605-18-1 | 26 | 0.62 a | 1.99–31.76 a |
| 62.00d | ||||
| 0.05–3.60 e | 0.51–26.58 e | |||
| diphenylethanedione | 134-81-6 | 6 | 1.50–7.34 e | 8.06–153.78 m |
| ethyl 2,4,6-trimethylbenzoate | 1754-55-8 | 4 | 5.53–44.54 e | |
| diethyl phenylphosphonate | 1754-49-0 | 4 | 5.16–24.09 e | |
| 1,3,5-trimethylbenzene | 108-67-8 | 4 | 14.55–63.48 c | |
| 2,4,6-trimethylphenol | 527-60-6 | 7 | 0.33–83.37 e | |
| 1.35 h | 1.93 h | |||
| benzoic acid | 65-85-0 | 20 | 38.58–67.23 c | 1.69–27.09 c |
| 0.77–10.79 e | ||||
| 2-hydroxy-2-methyl-1-phenylpropanone | 7473-98-5 | 1 | 17.91 e | |
| methyl-4-dimethyl aminobenzoate | 1202-25-1 | 7 | 0.56 c | 1.14–2.01 c |
| 5.86–14.49 e | 26.26–44.77 e | |||
| 4-diethylaminobenzoic acid | 5429-28-7 | 1 | 25.81 e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarsella, J.B.; Zhang, N.; Hartman, T.G. Identification and Migration Studies of Photolytic Decomposition Products of UV-Photoinitiators in Food Packaging. Molecules 2019, 24, 3592. https://doi.org/10.3390/molecules24193592
Scarsella JB, Zhang N, Hartman TG. Identification and Migration Studies of Photolytic Decomposition Products of UV-Photoinitiators in Food Packaging. Molecules. 2019; 24(19):3592. https://doi.org/10.3390/molecules24193592
Chicago/Turabian StyleScarsella, Joseph B., Nan Zhang, and Thomas G. Hartman. 2019. "Identification and Migration Studies of Photolytic Decomposition Products of UV-Photoinitiators in Food Packaging" Molecules 24, no. 19: 3592. https://doi.org/10.3390/molecules24193592





