A Time-Resolved Study on the Reactivity of Alcoholic Drinks with the Hydroxyl Radical
Abstract
1. Introduction
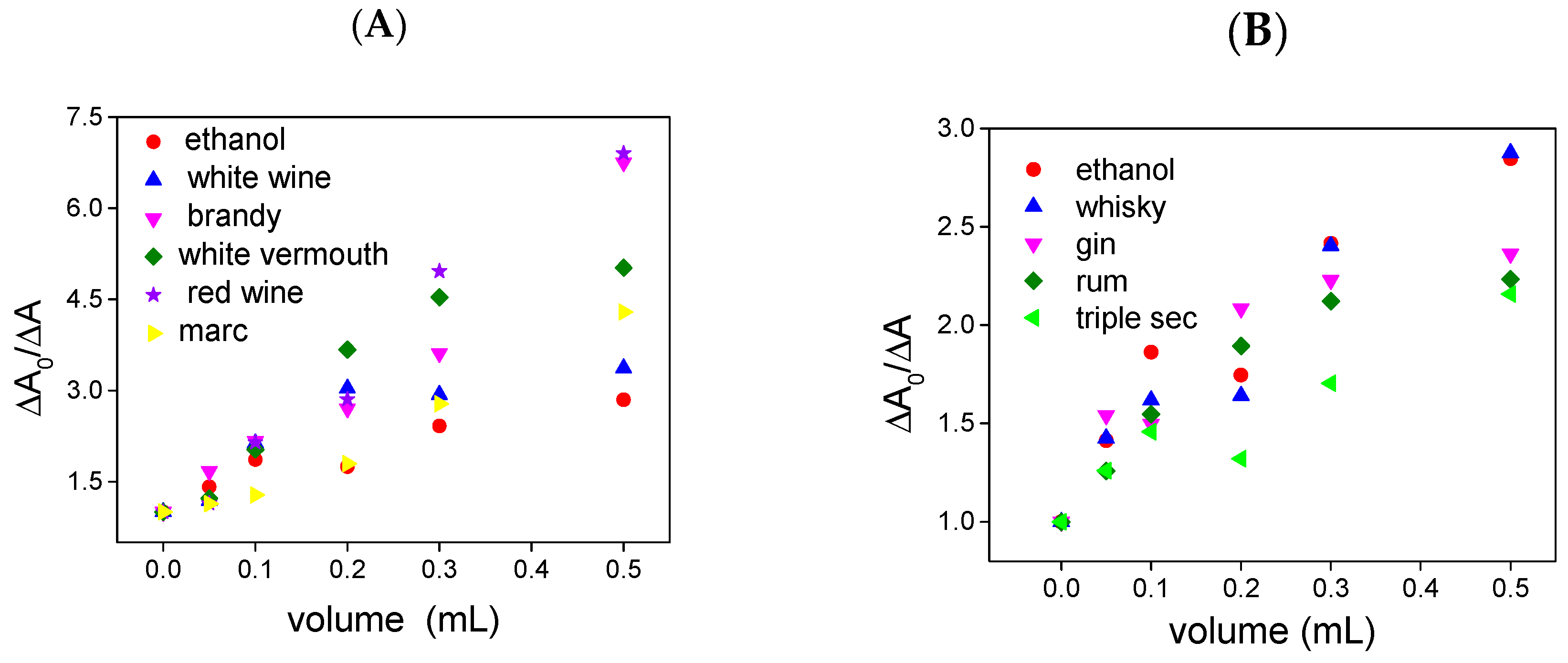
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Other Reagents
3.2. Photophysical Instrumentation
3.3. Kinetic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nathan, C.; Ding, A. SnapShot: Reactive oxygen intermediates (ROI). Cell 2010, 140. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Rodriguez-Muñiz, G.M.; Gomis, J.; Arques, A.; Amat, A.M.; Marin, M.L.; Miranda, M.A. Hydroxyl radical as an unlikely key intermediate in the photodegradation of emerging pollutants. Photochem. Photobiol. 2014, 90, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pehkonen, S.O.; Lin, C.-J. Degradation of monomethylmercury chloride by hydroxyl radicals in simulated natural waters. Water Res. 2003, 37, 2496–2504. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Finley, J.W.; Kong, A.-N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Antioxidants in foods: State of the science important to the food industry. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Overview of reactive oxygen species. In Singlet Oxygen: Applications in Biosciences and Nanosciences, Volume 1; The Royal Society of Chemistry: London, UK, 2016; pp. 1–21. [Google Scholar]
- Beal, M.F. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2003, 991, 120–131. [Google Scholar] [CrossRef]
- Čolak, E. New markers of oxidative damage to macromolecules. J. Med. Biochem. 2008, 27, 1–16. [Google Scholar] [CrossRef]
- Moreira, P.I.; Smith, M.A.; Zhu, X.; Honda, K.; Lee, H.G.; Aliev, G.; Perry, G. Oxidative damage and Alzheimer’s disease: Are antioxidant therapies useful? Drug News Perspect. 2005, 18, 13–19. [Google Scholar]
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Selected methods of extracting carotenoids, characterization, and health concerns: A review. J. Agric. Food Chem. 2018, 66, 5925–5947. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Sithisarn, P.; Carlsen, C.U.; Andersen, M.L.; Gritsanapan, W.; Skibsted, L.H. Antioxidative effects of leaves from Azadirachta species of different provenience. Food Chem. 2007, 104, 1539–1549. [Google Scholar] [CrossRef]
- Song, L.-L.; Liang, R.; Li, D.-D.; Xing, Y.-D.; Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. β-Carotene radical cation addition to green tea polyphenols. Mechanism of antioxidant antagonism in peroxidizing liposomes. J. Agric. Food Chem. 2011, 59, 12643–12651. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, Z.; Ohm, J.-B.; Schwarz, P.; Rao, J.; Chen, B. Improvement of the antioxidative activity of soluble phenolic compounds in chickpea by germination. J. Agric. Food Chem. 2018, 66, 6179–6187. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xue, X.; Li, H.; Apandi, S.N.; Tay-Chan, S.C.; Ong, S.P.; Tian, E.F. The relative antioxidant activity and steric structure of green tea catechins—A kinetic approach. Food Chem. 2018, 257, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Kanner, J.; Frankel, E.; Granit, R.; German, B.; Kinsella, J.E. Natural antioxidants in grapes and wines. J. Agric. Food Chem. 1994, 42, 64–69. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Niki, E.; Noguchi, N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life 2000, 50, 323–329. [Google Scholar] [CrossRef] [PubMed]
- DeMatteo, M.P.; Poole, J.S.; Shi, X.; Sachdeva, R.; Hatcher, P.G.; Hadad, C.M.; Platz, M.S. On the electrophilicity of hydroxyl radical: A laser flash photolysis and computational study. J. Am. Chem. Soc. 2005, 127, 7094–7109. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.S.; Shi, X.; Hadad, C.M.; Platz, M.S. Reaction of hydroxyl radical with aromatic hydrocarbons in nonaqueous solutions: A laser flash photolysis study in acetonitrile. J. Phys. Chem. A 2005, 109, 2547–2551. [Google Scholar] [CrossRef]
- Marin, M.L.; Lhiaubet-Vallet, V.; Santos-Juanes, L.; Soler, J.; Gomis, J.; Argues, A.; Miranda, M.A. A photophysical approach to investigate the photooxidation mechanism of pesticides: Hydroxyl radical versus electron transfer. Appl. Catal. B 2011, 103, 48–53. [Google Scholar] [CrossRef]
- Rodríguez-Muñiz, G.M.; Marin, M.L.; Lhiaubet-Vallet, V.; Miranda, M.A. Reactivity of nucleosides with a hydroxyl radical in non-aqueous medium. Chem. Eur. J. 2012, 18, 8024–8027. [Google Scholar] [CrossRef]
- Mitroka, S.; Zimmeck, S.; Troya, D.; Tanko, J.M. How solvent modulates hydroxyl radical reactivity in hydrogen atom abstractions. J. Am. Chem. Soc. 2010, 132, 2907–2913. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Maiani, G.; Ferro-Luzzi, A. Alcohol-free red wine enhances plasma antioxidant capacity in humans. J. Nutr. 1998, 128, 1003–1007. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 2001, 49, 5736–5742. [Google Scholar] [CrossRef]
- Frankel, E.N.; Kanner, J.; German, J.B.; Parks, E.; Kinsella, J.E. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 1993, 341, 454–457. [Google Scholar] [CrossRef]
- Ghiselli, A.; Nardini, M.; Baldi, A.; Scaccini, C. Antioxidant activity of different phenolic fractions separated from an Italian red wine. J. Agric. Food Chem. 1998, 46, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, T.L.; Koop, D.R. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem. Pharmacol. 1999, 57, 941–949. [Google Scholar] [CrossRef]
- Paceasciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef]
- Schneider, Y.; Vincent, F.; Duranton, B.; Badolo, L.; Gosse, F.; Bergmann, C.; Raul, F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 2000, 158, 85–91. [Google Scholar] [CrossRef]
- Li, D.D.; Han, R.M.; Liang, R.; Chen, C.H.; Lai, W.Z.; Zhang, J.P.; Skibsted, L.H. Hydroxyl radical reaction with trans-resveratrol: Initial carbon radical adduct formation followed by rearrangement to phenoxyl radical. J. Phys. Chem. B 2012, 116, 7154–7161. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Beverage | Relative kOH· | Relative Ethanol Content |
|---|---|---|
| Ethanol | 1 | 1 |
| Red wine a | 2.89 | 0.13 |
| White wine b | 1.42 | 0.12 |
| White vermouth c | 2.17 | 0.15 |
| Marc d | 1.47 | 0.30 |
| Brandy e | 2.61 | 0.36 |
| Triple sec f | 0.58 | 0.40 |
| Gin g | 0.86 | 0.38 |
| Whisky h | 0.97 | 0.40 |
| Rum i | 0.75 | 0.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Muñiz, G.M.; Miranda, M.A.; Marin, M.L. A Time-Resolved Study on the Reactivity of Alcoholic Drinks with the Hydroxyl Radical. Molecules 2019, 24, 234. https://doi.org/10.3390/molecules24020234
Rodriguez-Muñiz GM, Miranda MA, Marin ML. A Time-Resolved Study on the Reactivity of Alcoholic Drinks with the Hydroxyl Radical. Molecules. 2019; 24(2):234. https://doi.org/10.3390/molecules24020234
Chicago/Turabian StyleRodriguez-Muñiz, Gemma M., Miguel A. Miranda, and M. Luisa Marin. 2019. "A Time-Resolved Study on the Reactivity of Alcoholic Drinks with the Hydroxyl Radical" Molecules 24, no. 2: 234. https://doi.org/10.3390/molecules24020234
APA StyleRodriguez-Muñiz, G. M., Miranda, M. A., & Marin, M. L. (2019). A Time-Resolved Study on the Reactivity of Alcoholic Drinks with the Hydroxyl Radical. Molecules, 24(2), 234. https://doi.org/10.3390/molecules24020234








