Notoginsenoside R1 Protects db/db Mice against Diabetic Nephropathy via Upregulation of Nrf2-Mediated HO-1 Expression
Abstract
1. Introduction
2. Results
2.1. The Impact of NGR1 on Fasting Blood Glucose (FBG), Total Cholesterol (TCH), and Triacylglycerol (TG) Levels in db/db Mice
2.2. NGR1 Treatment Protected Kidney Function in the db/db Mice
2.3. The Impact of NGR1 on Histopathological Changes
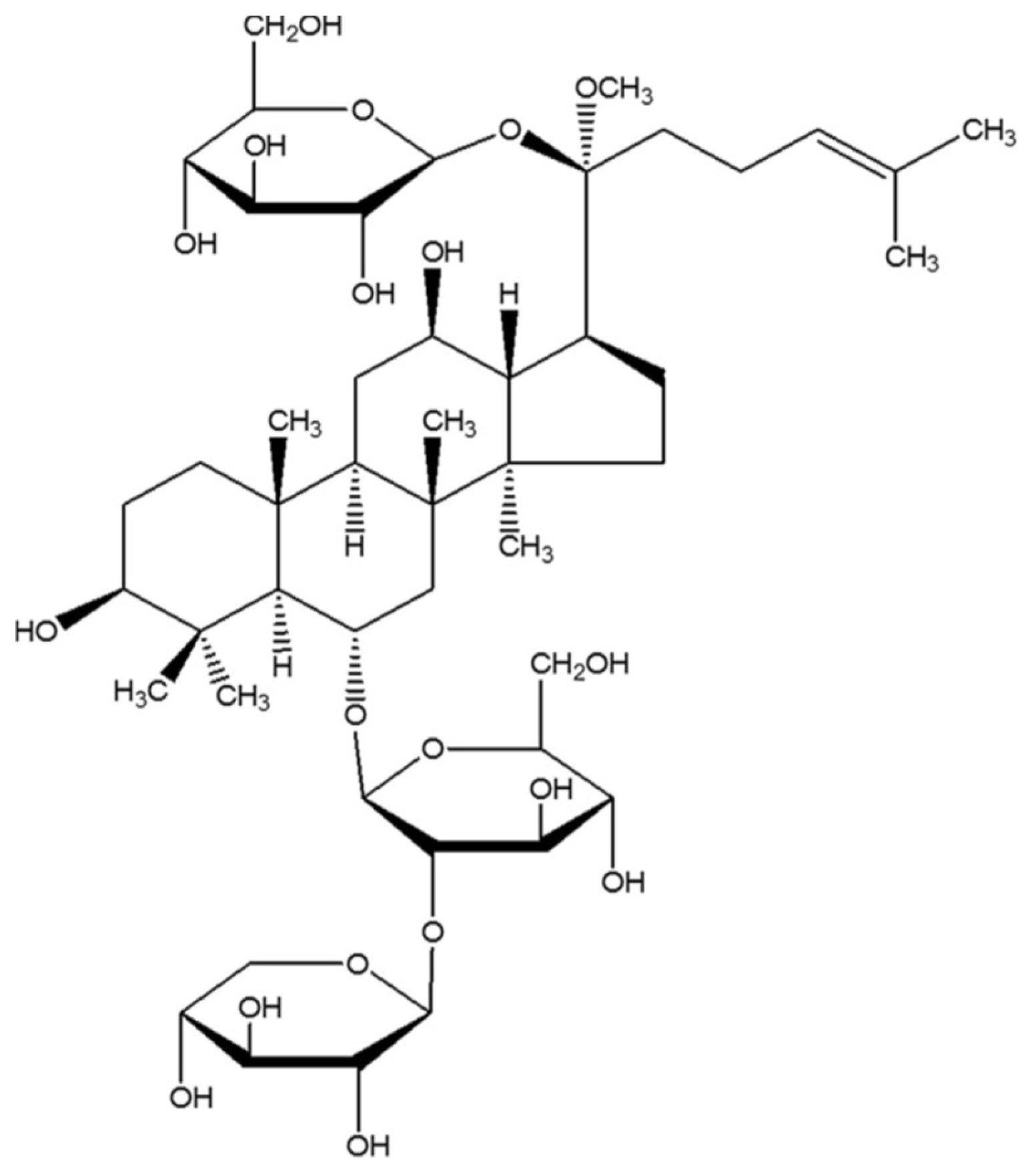
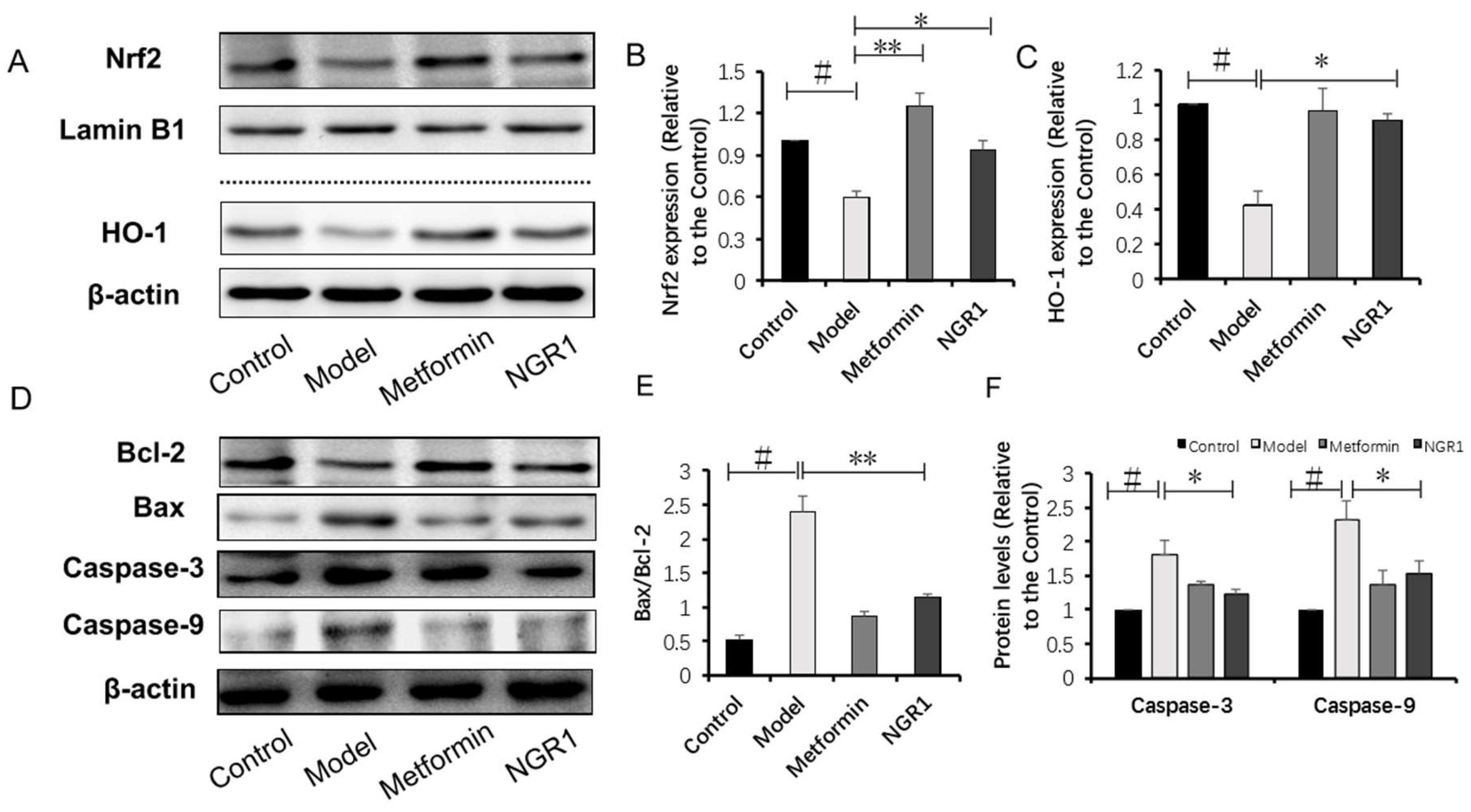
2.4. NGR1 Inhibits Diabetes-Induced Apoptosis via Upregulation of Nrf2-Mediated HO-1 Expression
2.5. NGR1 Inhibits AGE-Induced HK-2 Cell Death
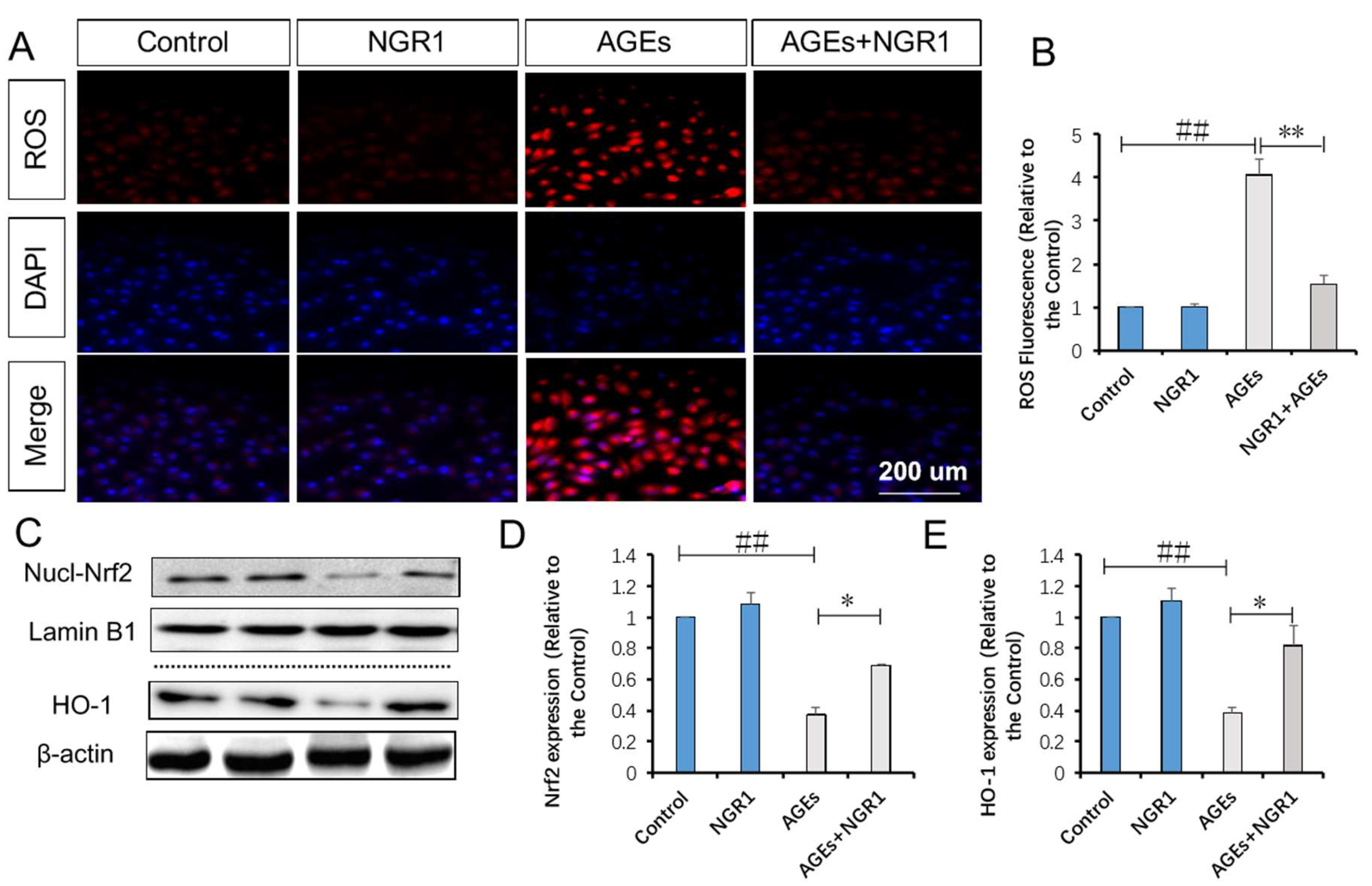
2.6. NGR1 Suppresses AGE-Induced Mitochondrial Superoxide Production in HK-2 Cells via Upregulation of Nrf2-Mediated HO-1
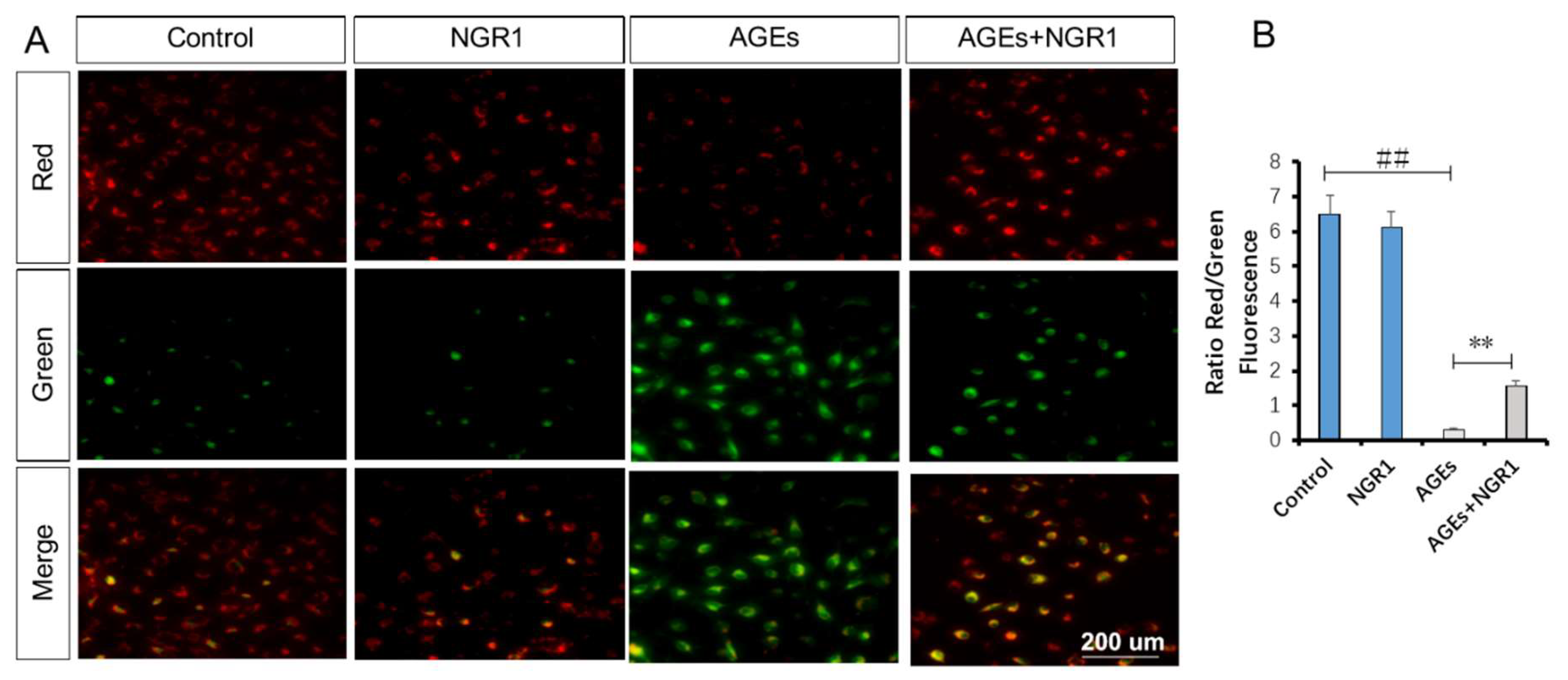
2.7. NGR1 Alleviates AGE-Induced Mitochondrial Injury in HK-2 Cells
2.8. NGR1 Inhibits AGE-Induced Cell Apoptosis in HK-2 Cells
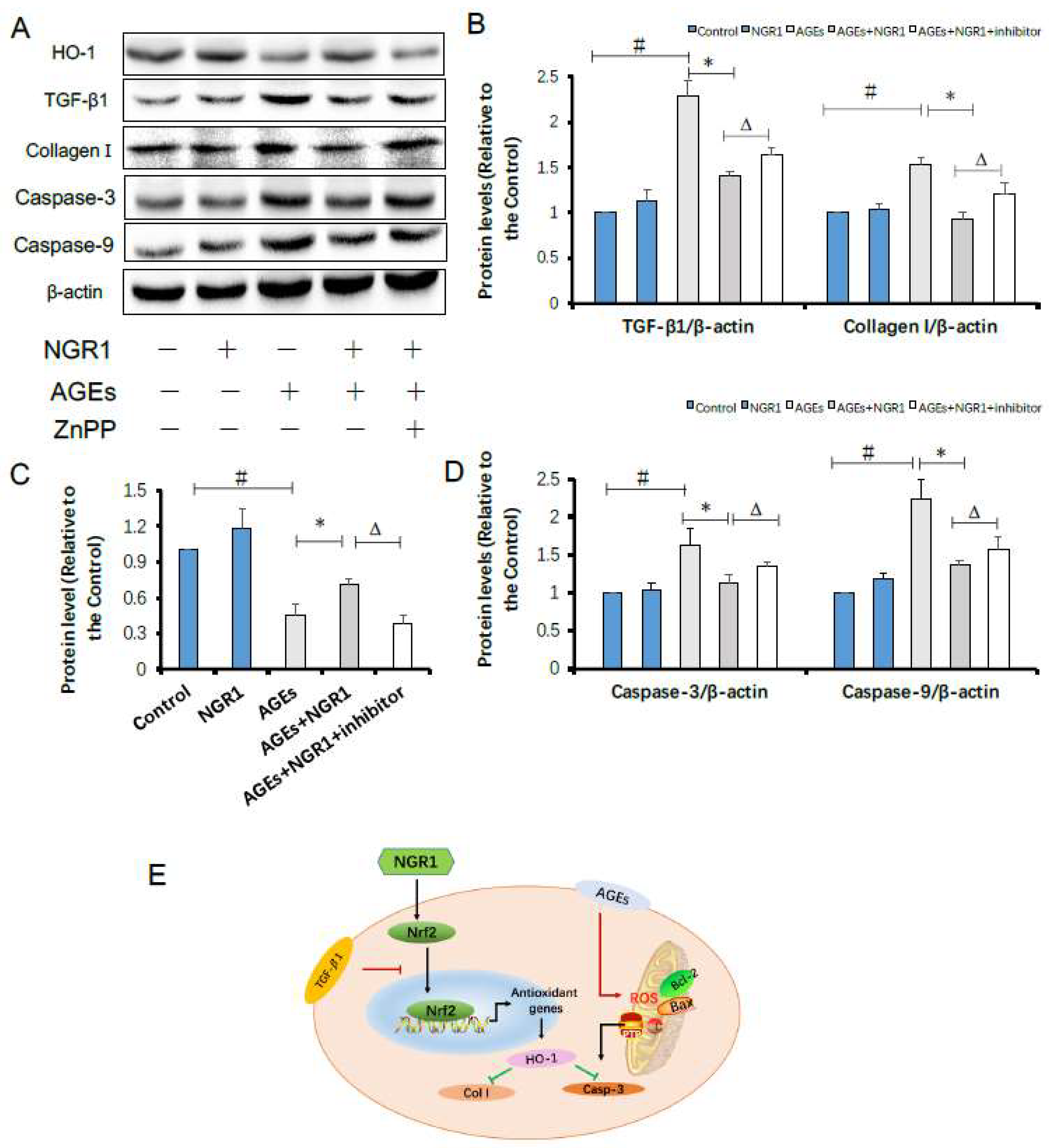
2.9. HO-1 Inhibitor Neutralizes the Protective Effects of NGR1 by Resisting Its Inhibition of Apoptosis and the TGF-β Pathway
2.10. Discussion
3. Materials and Methods
3.1. Reagent and Materials
3.2. Preparation of AGE/Bovine Serum Albumin (BSA)
3.3. Animal and Groups
3.4. Experimental Protocol
3.5. Fasting Blood Glucose, and Serum TCH and TG Levels
3.6. Serum β2-Microglobulin, Blood Urea Nitrogen (BUN), Creatinine (CR), AGE, and Urinary Albumin Measurements
3.7. Histopathological Examination
3.8. Terminal Deoxynucleotidyl Transferase (TdT) dUTP Nick-End Labeling (TUNEL) Staining
3.9. Immunohistochemistry (ICH) Staining
3.10. Cell Culture and Treatment
3.11. Cell Viability Assay
3.12. Detection of Mitochondrial Superoxides (ROS)
3.13. Detection of Mitochondrial Membrane Potential (∆Ψm)
3.14. Flow Cytometric Detection of Apoptosis
3.15. Western Blotting Analysis
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, P.; Zhang, X.; Brown, J.; Vistisen, D.; Sicree, R.; Shaw, J.; Nichols, G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 293–301. [Google Scholar] [CrossRef]
- Tervaert, T.W.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef]
- Taft, J.L.; Nolan, C.J.; Yeung, S.P.; Hewitson, T.D.; Martin, F.I. Clinical and histological correlations of decline in renal function in diabetic patients with proteinuria. Diabetes 1994, 43, 1046–1051. [Google Scholar] [CrossRef]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 24, 18–32. [Google Scholar] [CrossRef]
- Yamagishi, S.; Matsui, T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid. Med. Cell. Longev. 2010, 3, 101–108. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E.; Oldfield, M.D.; Thomas, M.C. Role of advanced glycation end products in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, S254–S258. [Google Scholar] [CrossRef]
- Dounousi, E.; Duni, A.; Leivaditis, K.; Vaios, V.; Eleftheriadis, T.; Liakopoulos, V. Improvements in the Management of Diabetic Nephropathy. Rev. Diabet. Stud. 2015, 12, 119–133. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Kaur, N.; Kishore, L.; Singh, R. Therapeutic effect of Linum usitatissimum L. in STZ-nicotinamide induced diabetic nephropathy via inhibition of AGE's and oxidative stress. J. Food Sci. Technol. 2017, 54, 408–421. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Yang, S.L.; Wang, J.Y.; Ye, M.; Zhuo, X.Y.; Wang, L.T.; Chen, H.; Zhang, H.; Yang, L. Irbesartan attenuates advanced glycation end products-mediated damage in diabetes-associated osteoporosis through the AGEs/RAGE pathway. Life Sci. 2018, 205, 184–192. [Google Scholar] [CrossRef]
- Kishore, L.; Kaur, N.; Singh, R. Renoprotective effect of Bacopa monnieri via inhibition of advanced glycation end products and oxidative stress in STZ-nicotinamide-induced diabetic nephropathy. Ren. Fail. 2016, 38, 1528–1544. [Google Scholar] [CrossRef] [PubMed]
- Isermann, B.; Vinnikov, I.A.; Madhusudhan, T.; Herzog, S.; Kashif, M.; Blautzik, J.; Corat, M.A.; Zeier, M.; Blessing, E.; Oh, J.; et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 2007, 13, 1349–1358. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am. J. Nephrol. 2017, 45, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Min, X.; Xu, X.; Du, B.; Luo, P. Role of nuclear factor erythroid 2-related factor 2 in diabetic nephropathy. J. Diabetes Res. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ning, B.; Cui, J.; Zhang, T.; Chen, Y. miR-29c is implicated in the cardioprotective activity of Panax notoginseng saponins against isoproterenol-induced myocardial fibrogenesis. J. Ethnopharmacol. 2017, 198, 1–4. [Google Scholar] [CrossRef]
- Son, H.Y.; Han, H.S.; Jung, H.W.; Park, Y.K. Panax notoginseng attenuates the infarct volume in rat ischemic brain and the inflammatory response of microglia. J. Pharmacol. Sci. 2009, 109, 368–379. [Google Scholar] [CrossRef]
- Meng, X.; Sun, G.; Ye, J.; Xu, H.; Wang, H.; Sun, X. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: A novel mechanism of Nrf2/ARE signaling activation. Free Radic. Res. 2014, 48, 445–460. [Google Scholar] [CrossRef]
- Ma, B.; Meng, X.; Wang, J.; Sun, J.; Ren, X.; Qin, M.; Sun, J.; Sun, G.; Sun, X. Notoginsenoside R1 attenuates amyloid-beta-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 2014, 22, 151–159. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, G.; Luo, Y.; Wang, M.; Chen, R.; Zhang, J.; Ai, Q.; Xing, N.; Sun, X. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress-related signaling pathways. Sci. Rep. 2016, 6, 21730. [Google Scholar] [CrossRef]
- Tian, N.; Gao, Y.; Wang, X.; Wu, X.; Zou, D.; Zhu, Z.; Han, Z.; Wang, T.; Shi, Y. Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy. Drug Des. Dev. Ther. 2018, 12, 2195–2211. [Google Scholar] [CrossRef]
- Wang, X.B.; Zhu, H.; Song, W.; Su, J.H. Gremlin regulates podocyte apoptosis via transforming growth factor-beta (TGF-beta) pathway in diabetic nephropathy. Med. Sci. Monit. 2018, 24, 183–189. [Google Scholar] [CrossRef]
- Lu, H.; Li, Y.; Zhang, T.; Liu, M.; Chi, Y.; Liu, S.; Shi, Y. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upregulating heme oxygenase-1 (HO-1) expression. Med. Sci. Monit. 2017, 23, 4067–4076. [Google Scholar] [CrossRef]
- Meng, X.; Wang, M.; Wang, X.; Sun, G.; Ye, J.; Xu, H.; Sun, X. Suppression of NADPH oxidase- and mitochondrion-derived superoxide by notoginsenoside R1 protects against cerebral ischemia-reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways. Free Radic. Res. 2014, 48, 823–838. [Google Scholar] [CrossRef]
- Yang, Y.C.; Tsai, C.Y.; Chen, C.L.; Kuo, C.H.; Hou, C.W.; Cheng, S.Y.; Aneja, R.; Huang, C.Y.; Kuo, W.W. PKCdelta activation is involved in ros-mediated mitochondrial dysfunction and apoptosis in cardiomyocytes exposed to advanced glycation end products (ages). Aging Dis. 2018, 9, 647–663. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress? Free Radic. Biol. Med. 2018, 116, 50–63. [Google Scholar] [CrossRef]
- Jha, J.C.; Ho, F.; Dan, C.; Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. 2018, 132, 1811–1836. [Google Scholar] [CrossRef]
- Chou, S.T.; Tseng, S.T. Oxidative stress markers in type 2 diabetes patients with diabetic nephropathy. Clin. Exp. Nephrol. 2017, 21, 283–292. [Google Scholar] [CrossRef]
- Striker, L.J.; Doi, T.; Elliot, S.; Striker, G.E. The contribution of glomerular mesangial cells to progressive glomerulosclerosis. Semin. Nephrol. 1989, 9, 318–328. [Google Scholar]
- Stokes, M.B.; Hudkins, K.L.; Zaharia, V.; Taneda, S.; Alpers, C.E. Up-regulation of extracellular matrix proteoglycans and collagen type I in human crescentic glomerulonephritis. Kidney Int. 2001, 59, 532–542. [Google Scholar] [CrossRef]
- Fu, J.; Lee, K.; Chuang, P.Y.; Liu, Z.; He, J.C. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am. J. Physiol. Ren. Physiol. 2015, 308, F287–F297. [Google Scholar] [CrossRef]
- Xu, H.L.; Wang, X.T.; Cheng, Y.; Zhao, J.G.; Zhou, Y.J.; Yang, J.J.; Qi, M.Y. Ursolic acid improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocin-induced rats. Biomed. Pharmacother. 2018, 105, 915–921. [Google Scholar] [CrossRef]
- Pourghasem, M.; Shafi, H.; Babazadeh, Z. Histological changes of kidney in diabetic nephropathy. Caspian J. Intern. Med. 2015, 6, 120–127. [Google Scholar]
- Mason, R.M.; Wahab, N.A. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1358–1373. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakamura, T.; Noble, N.A.; Ruoslahti, E.; Border, W.A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1993, 90, 1814–1818. [Google Scholar] [CrossRef]
- Ono, H.; Abe, H.; Sakurai, A.; Ochi, A.; Tominaga, T.; Tamaki, M.; Kishi, S.; Murakami, T.; Nagai, K.; Kohashi, M.; et al. Novel Interplay Between Smad1 and Smad3 Phosphorylation via AGE Regulates the Progression of Diabetic Nephropathy. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Kaur, N.; Kishore, L.; Singh, R. Dillenia indica L. attenuates diabetic nephropathy via inhibition of advanced glycation end products accumulation in STZ-nicotinamide induced diabetic rats. J. Tradit. Complement. Med. 2018, 8, 226–238. [Google Scholar] [CrossRef]
- Gawandi, S.; Gangawane, S.; Chakrabarti, A.; Kedare, S.; Bantwal, K.; Wadhe, V.; Kulkarni, A.; Kulkarni, S.; Rajan, M.G.R. A Study of Microalbuminuria (MAU) and Advanced Glycation End Products (AGEs) Levels in Diabetic and Hypertensive Subjects. Indian J. Clin. Biochem. 2018, 33, 81–85. [Google Scholar] [CrossRef]
- Chilelli, N.C.; Burlina, S.; Lapolla, A. AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: A “glycoxidation-centric” point of view. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 913–919. [Google Scholar] [CrossRef]
- Fleming, T.; Cuny, J.; Nawroth, G.; Djuric, Z.; Humpert, P.M.; Zeier, M.; Bierhaus, A.; Nawroth, P.P. Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates? Diabetologia 2012, 55, 1151–1155. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Bassiouni, Y.A.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mahmoud, A.M. Simvastatin ameliorates diabetic nephropathy by attenuating oxidative stress and apoptosis in a rat model of streptozotocin-induced type 1 diabetes. Biomed. Pharmacother. 2018, 105, 290–298. [Google Scholar] [CrossRef]
- Kuwabara, A.; Satoh, M.; Tomita, N.; Sasaki, T.; Kashihara, N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia 2010, 53, 2056–2065. [Google Scholar] [CrossRef]
- Chen, J.; Jing, J.; Yu, S.; Song, M.; Tan, H.; Cui, B.; Huang, L. Advanced glycation endproducts induce apoptosis of endothelial progenitor cells by activating receptor RAGE and NADPH oxidase/JNK signaling axis. Am. J. Transl. Res. 2016, 8, 2169–2178. [Google Scholar]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Diaz, A.G. Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef]
- Mao, C.Y.; Lu, H.B.; Kong, N.; Li, J.Y.; Liu, M.; Yang, C.Y.; Yang, P. Levocarnitine protects H9c2 rat cardiomyocytes from H2O2-induced mitochondrial dysfunction and apoptosis. Int. J. Med. Sci. 2014, 11, 1107–1115. [Google Scholar] [CrossRef]
- Sobhan, P.K.; Seervi, M.; Deb, L.; Varghese, S.; Soman, A.; Joseph, J.; Mathew, K.A.; Raghu, G.; Thomas, G.; Sreekumar, E.; et al. Calpain and reactive oxygen species targets Bax for mitochondrial permeabilisation and caspase activation in zerumbone induced apoptosis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Hosseinzadeh, L.; Behravan, J.; Mosaffa, F.; Bahrami, G.; Bahrami, A.; Karimi, G. Curcumin potentiates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through generation of reactive oxygen species. Food Chem. Toxicol. 2011, 49, 1102–1109. [Google Scholar] [CrossRef]
- Liu, Y.; Huo, Z.; Yan, B.; Lin, X.; Zhou, Z.N.; Liang, X.; Zhu, W.; Liang, D.; Li, L.; Liu, Y.; et al. Prolyl hydroxylase 3 interacts with Bcl-2 to regulate doxorubicin-induced apoptosis in H9c2 cells. Biochem. Biophys. Res. Commun. 2010, 401, 231–237. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, S.; Wang, B.; Du, F.; Hu, C.; Li, H.; Feng, Y.; Zhu, R.; Mo, M.; Cao, Y.; et al. 17β-Estradiol up-regulates Nrf2 via PI3K/AKT and estrogen receptor signaling pathways to suppress light-induced degeneration in rat retina. Neuroscience 2015, 304, 328–339. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.Y.; Zhang, C.Y.; Zhang, X.L.; Ye, J.X.; Kuang, S.H.; Sun, G.B.; Sun, X.B. Notoginsenoside R1 Protects Against Diabetic Cardiomyopathy Through Activating Estrogen Receptor α and Its Downstream Signaling. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.Y.; Jiang, Q.; Li, K.R.; Zhao, Y.X.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef]
- Ye, F.; Li, X.; Li, L.; Yuan, J.; Chen, J. t-BHQ Provides Protection against Lead Neurotoxicity via Nrf2/HO-1 Pathway. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Koo, Y.C.; Pyo, M.C.; Nam, M.H.; Hong, C.O.; Yang, S.Y.; Lee, K.W. Chebulic acid prevents hepatic fibrosis induced by advanced glycation end-products in LX-2 cell by modulating Nrf2 translocation via ERK pathway. Toxicol. In Vitro 2016, 34, 8–15. [Google Scholar] [CrossRef]
- Huang, K.; Huang, J.; Xie, X.; Wang, S.; Chen, C.; Shen, X.; Liu, P.; Huang, H. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic. Biol. Med. 2013, 65, 528–540. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, Q.; Chen, Y.; Pan, R.; Kuang, S.; Liu, G.; Sun, G.; Sun, X. Myricitrin Alleviates Oxidative Stress-induced Inflammation and Apoptosis and Protects Mice against Diabetic Cardiomyopathy. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Sun, B.; Xiao, J.; Sun, X.B.; Wu, Y. Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice: An insight into oestrogen receptor activation and PI3K/Akt signalling. Br. J. Pharmacol. 2013, 168, 1758–1770. [Google Scholar] [CrossRef]
Sample Availability: Notoginsenoside R1 is available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, X.; Zhang, C.; Shen, Q.; Sun, G.; Sun, X. Notoginsenoside R1 Protects db/db Mice against Diabetic Nephropathy via Upregulation of Nrf2-Mediated HO-1 Expression. Molecules 2019, 24, 247. https://doi.org/10.3390/molecules24020247
Zhang B, Zhang X, Zhang C, Shen Q, Sun G, Sun X. Notoginsenoside R1 Protects db/db Mice against Diabetic Nephropathy via Upregulation of Nrf2-Mediated HO-1 Expression. Molecules. 2019; 24(2):247. https://doi.org/10.3390/molecules24020247
Chicago/Turabian StyleZhang, Bin, Xuelian Zhang, Chenyang Zhang, Qiang Shen, Guibo Sun, and Xiaobo Sun. 2019. "Notoginsenoside R1 Protects db/db Mice against Diabetic Nephropathy via Upregulation of Nrf2-Mediated HO-1 Expression" Molecules 24, no. 2: 247. https://doi.org/10.3390/molecules24020247
APA StyleZhang, B., Zhang, X., Zhang, C., Shen, Q., Sun, G., & Sun, X. (2019). Notoginsenoside R1 Protects db/db Mice against Diabetic Nephropathy via Upregulation of Nrf2-Mediated HO-1 Expression. Molecules, 24(2), 247. https://doi.org/10.3390/molecules24020247




