Thiodiketopiperazines Produced by Penicillium crustosum and Their Activities to Promote Gastrointestinal Motility
Abstract
:1. Introduction
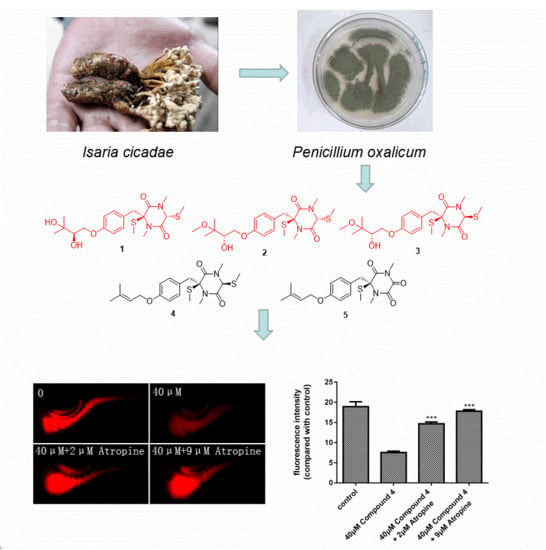
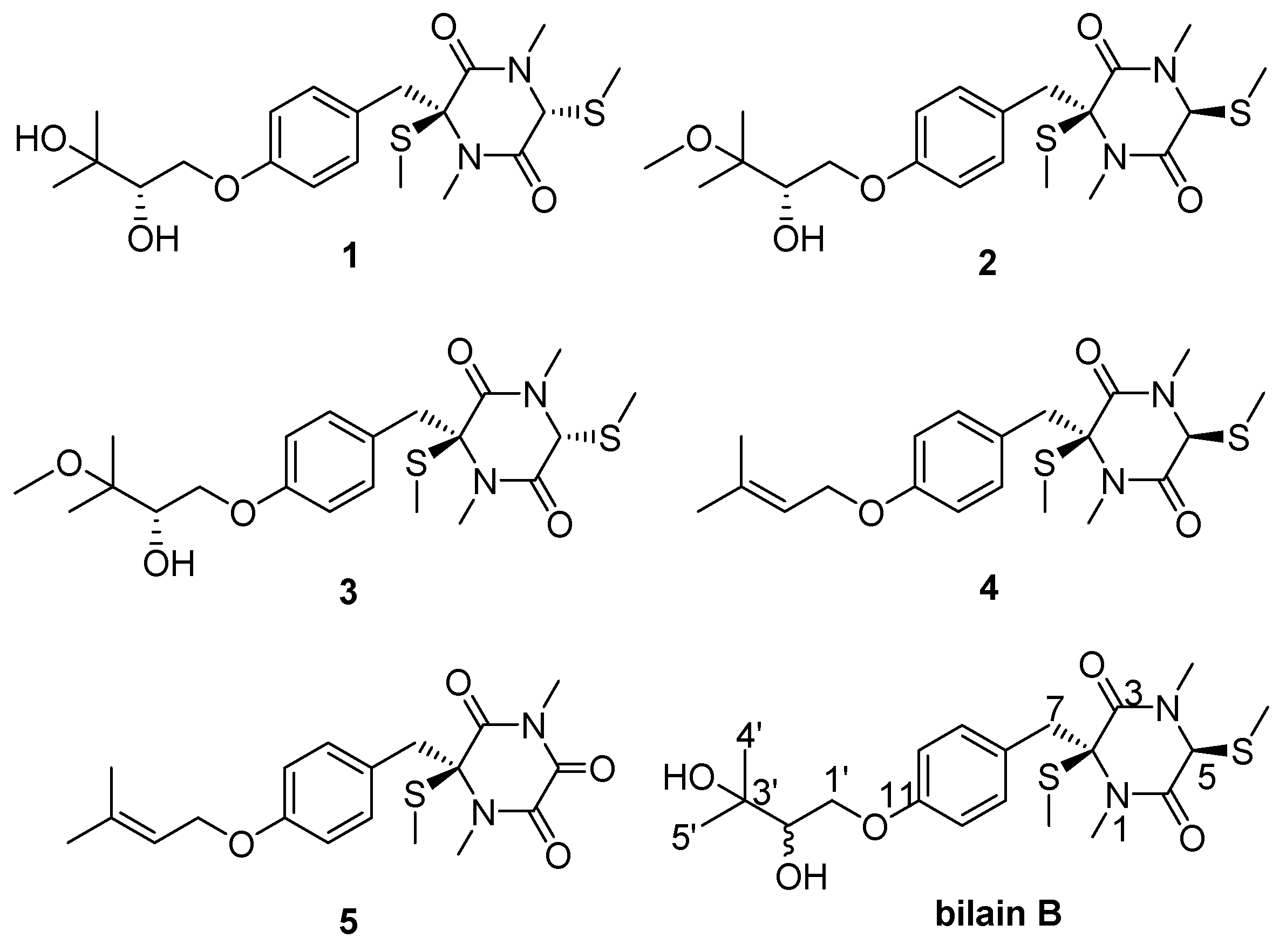
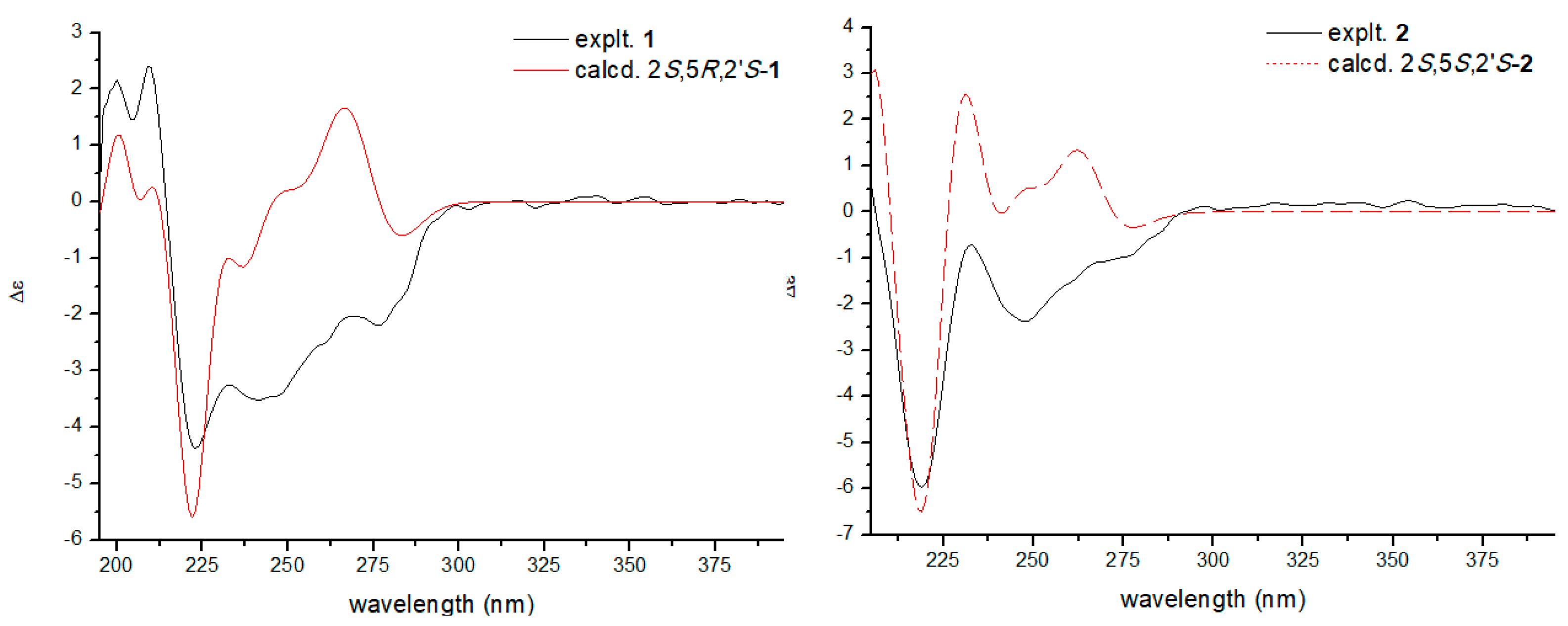
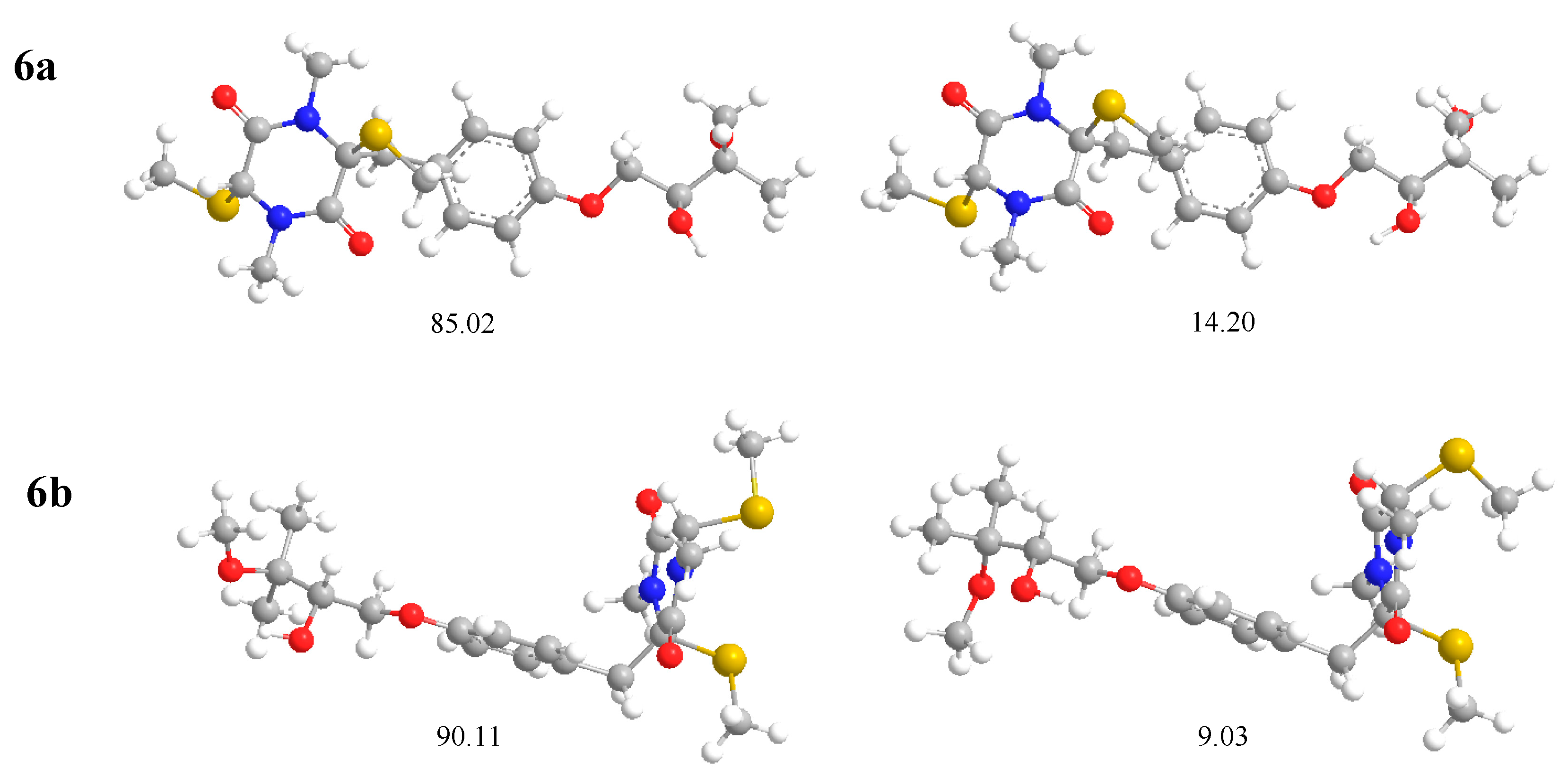
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Culture
3.3. Fractionation and Isolation
3.4. Preparation of the (S)- and (R)-MTPA Ester Derivatives of 1−3
- 1H NMR data (400 MHz, C5D5N) for (S-MTPA) ester of 1: δH 4.67 (1H, dd, J = 10.7, 2.1 Hz H-1′a), 4.29 (2H, dd, J = 10.7, 9.0 Hz, H-1′b), 1.51 (3H, s, H-4′), 1.51 (3H, s, H-5′).
- 1H NMR data (400 MHz, C5D5N) for (R-MTPA) ester of 1: δH 4.82 (1H, dd J = 10.8, 2.0 Hz, H-1′a), 4.47 (2H, dd, J = 10.7, 9.1 Hz, H-1′b), 1.44 (3H, s, H-4′), 1.40 (3H, s, H-5′).
- 1H NMR data (400 MHz, C5D5N) for (S-MTPA) ester of 2: δH 4.37 (1H, dd, J = 10.6, 2.2 Hz H-1′a), 4.12 (2H, dd, J = 10.7, 9.0 Hz, H-1′b), 1.28 (3H, s, H-4′), 1.26 (3H, s, H-5′).
- 1H NMR data (400 MHz, C5D5N) for (R-MTPA) ester of 2: δH 4.48 (1H, dd J = 10.7, 2.1 Hz, H-1′a), 4.27 (2H, dd, J = 10.7, 9.1 Hz, H-1′b), 1.19 (3H, s, H-4′), 1.17 (3H, s, H-5′).
- 1H NMR data (400 MHz, C5D5N) for (S-MTPA) ester of 3: δH 4.41 (1H, dd, J = 10.7, 2.1 Hz H-1′a), 4.15 (2H, dd, J = 10.7, 9.0 Hz, H-1′b), 1.26 (3H, s, H-4′), 1.24 (3H, s, H-5′).
- 1H NMR data (400 MHz, C5D5N) for (R-MTPA) ester of 3: δH 4.46 (1H, dd J = 10.7, 2.0 Hz, H-1′a), 4.25 (2H, dd, J = 10.7, 9.0 Hz, H-1′b), 1.17 (3H, s, H-4′), 1.16 (3H, s, H-5′).
3.5. Computational Details
3.6. Zebrafish Culture
3.7. Gastrointestinal Motility of Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskii, A.G.; Zhelifonova, V.P.; Antipova, T.V. Fungi of the genus Penicillium as producers of physiologically active compounds. Appl. Biochem. Microbiol. 2013, 49, 1–10. [Google Scholar] [CrossRef]
- Su, S.S.; Song, A.H.; Chen, G.; Wang, H.F.; Li, Z.Q.; Pei, Y.H. Two new indole-diterpenoids from the fungus Penicillium crustosum YN-HT-15. J. Asian Nat. Prod. Res. 2014, 16, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Trimble, L.A.; Sumarah, M.W.; Blackwell, B.A.; Wrona, M.D.; Miller, J.D. Characterization of (16R) and (16S)-hydroxyroquefortine C; diastereomeric metabolites from Penicillium crustosum DAOM 215343. Tetrahedron Lett. 2012, 53, 956–958. [Google Scholar] [CrossRef]
- Wang, H.F.; Chen, G.; Liu, Y.P.; Hua, H.M.; Bai, J.; Pei, Y.H. 1H and 13C NMR assignments of two new piperazine-trione from the fungus Penicillium crustosum YN-HT-15. Magn. Reson. Chem. 2015, 53, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.W.; Ma, H.Y.; Zhu, T.J.; Li, J.; Gu, Q.Q.; Li, D.H. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2. Tetrahedron 2012, 68, 9745–9749. [Google Scholar] [CrossRef]
- Liu, Y.P.; Chen, G.; Wang, H.F.; Zhang, X.L.; Pei, Y.H. Two new compounds from the fungus Penicillium crustosum YN-HT-15. J. Asian Nat. Prod. Res. 2014, 16, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Huang, X.; Cole, T. A high-throughput method for predicting drug effects on gut transit time using larval zebrafish. J. Pharmacol. Toxicol. Methods 2015, 76, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, I.; Eisen, J. Development of the zebrafish enteric nervous system. Method Cell Biol. 2011, 101, 143–160. [Google Scholar]
- Fleming, A.; Jankowski, J.; Goldsmith, P. In vivo analysis of gut function and disease changes in a zebrafish larvae model of inflammatory bowel disease: A feasibility study. Inflamm. Bowel Dis. 2010, 16, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Stewart, M.; Ratnayake, R.; Lacey, E.; Gill, J.H. Citromycetins and bilains A–C: New Aromatic polyketides and diketopiperazines from Australian marine-derived and Terrestrial penicillium spp. J. Nat. Prod. 2007, 70, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, D.O.; Borges, W.S.; Vieira, N.J.; de Oliveira, L.F.; da Silva, C.H.T.P.; Lopes, N.P.; Dias, L.G.; Duran-Patron, R.; Collado, I.G.; Pupo, M.T. Diketopiperazines produced by endophytic fungi found in association with two Asteraceae species. Phytochemistry 2010, 71, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis Version 1.61; University of Würzburg: Würzburg, Germany, 2013. [Google Scholar]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |



| Position | 1 | δC | 2 | δC | 3 | δC |
|---|---|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | ||||
| 1 | - | - | - | - | - | - |
| 2 | - | 76.9 | - | 75.1 | - | 76.5 |
| 3 | - | 165.4 | - | 164.4 | - | 165.0 |
| 4 | - | - | - | - | - | - |
| 5 | 4.92, s | 66.0 | 4.19, s | 65.1 | 4.59, s | 65.8 |
| 6 | - | 165.2 | - | 165.1 | - | 164.8 |
| 7 | 3.14, d (14.0) 3.58, d (14.0) | 41.0 | 3.07, d (14.0) 3.53, d (14.0) | 42.0 | 3.06, d (14.1) 3.65, d (14.1) | 40.7 |
| 8 | - | 128.1 | - | 126.5 | - | 126.9 |
| 9,13 | 7.11, d (8.7) | 132.5 | 6.97, d (8.7) | 130.8 | 7.06, d (8.7) | 131.6 |
| 10,12 | 6.85, d (8.7) | 115.8 | 6.79, d (8.7) | 114.7 | 6.82, d (8.7) | 115.3 |
| 11 | - | 159.6 | - | 158.3 | - | 158.6 |
| 1′ | 4.23, dd (10.0, 3.0) 3.89, dd (10.0, 7.7) | 70.7 | 4.07, dd (8.8, 3.2) 3.90, dd (14.1, 5.3) | 69.1 | 4.08, dd (9.4, 3.1) 3.91, dd (9.4, 7.7) | 69.2 |
| 2′ | 3.72, td (4.5, 2.2) | 77.1 | 3.85-3.88, m | 75.4 | 3.85, dd (7.6, 3.1) | 75.3 |
| 3′ | - | 71.9 | - | 76.2 | - | 76.2 |
| 4′ | 1.23, s | 26.7 | 1.23, s | 21.0 | 1.22, s | 21.0 |
| 5′ | 1.21, s | 25.6 | 1.22, s | 20.8 | 1.21, s | 20.7 |
| 1-Me | 3.20, s | 30.6 | 3.25, s | 30.3 | 3.04, s | 33.5 |
| 2-SMe | 1.95, s | 12.6 | 2.16, s | 13.7 | 1.95, s | 12.7 |
| 4-Me | 3.01, s | 33.2 | 2.95, s | 33.6 | 3.24, s | 30.7 |
| 5-SMe | 1.58, s | 13.7 | 2.29 | 16.5 | 1.66, s | 14.2 |
| 3’-OMe | - | - | 3.26, s | 49.4 | 3.25, s | 49.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Yang, J.; Qiu, L.; Feng, D.; Ju, F.; Tan, L.; Li, Y.-Z.; Gu, Y.-C.; Zhang, Z.; Guo, D.-L.; et al. Thiodiketopiperazines Produced by Penicillium crustosum and Their Activities to Promote Gastrointestinal Motility. Molecules 2019, 24, 299. https://doi.org/10.3390/molecules24020299
He X, Yang J, Qiu L, Feng D, Ju F, Tan L, Li Y-Z, Gu Y-C, Zhang Z, Guo D-L, et al. Thiodiketopiperazines Produced by Penicillium crustosum and Their Activities to Promote Gastrointestinal Motility. Molecules. 2019; 24(2):299. https://doi.org/10.3390/molecules24020299
Chicago/Turabian StyleHe, Xin, Jing Yang, Ling Qiu, Dan Feng, Feng Ju, Lu Tan, Yu-Zhi Li, Yu-Cheng Gu, Zhen Zhang, Da-Le Guo, and et al. 2019. "Thiodiketopiperazines Produced by Penicillium crustosum and Their Activities to Promote Gastrointestinal Motility" Molecules 24, no. 2: 299. https://doi.org/10.3390/molecules24020299
APA StyleHe, X., Yang, J., Qiu, L., Feng, D., Ju, F., Tan, L., Li, Y.-Z., Gu, Y.-C., Zhang, Z., Guo, D.-L., & Deng, Y. (2019). Thiodiketopiperazines Produced by Penicillium crustosum and Their Activities to Promote Gastrointestinal Motility. Molecules, 24(2), 299. https://doi.org/10.3390/molecules24020299