Partial Characterization, the Immune Modulation and Anticancer Activities of Sulfated Polysaccharides from Filamentous Microalgae Tribonema sp.
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization of TSP
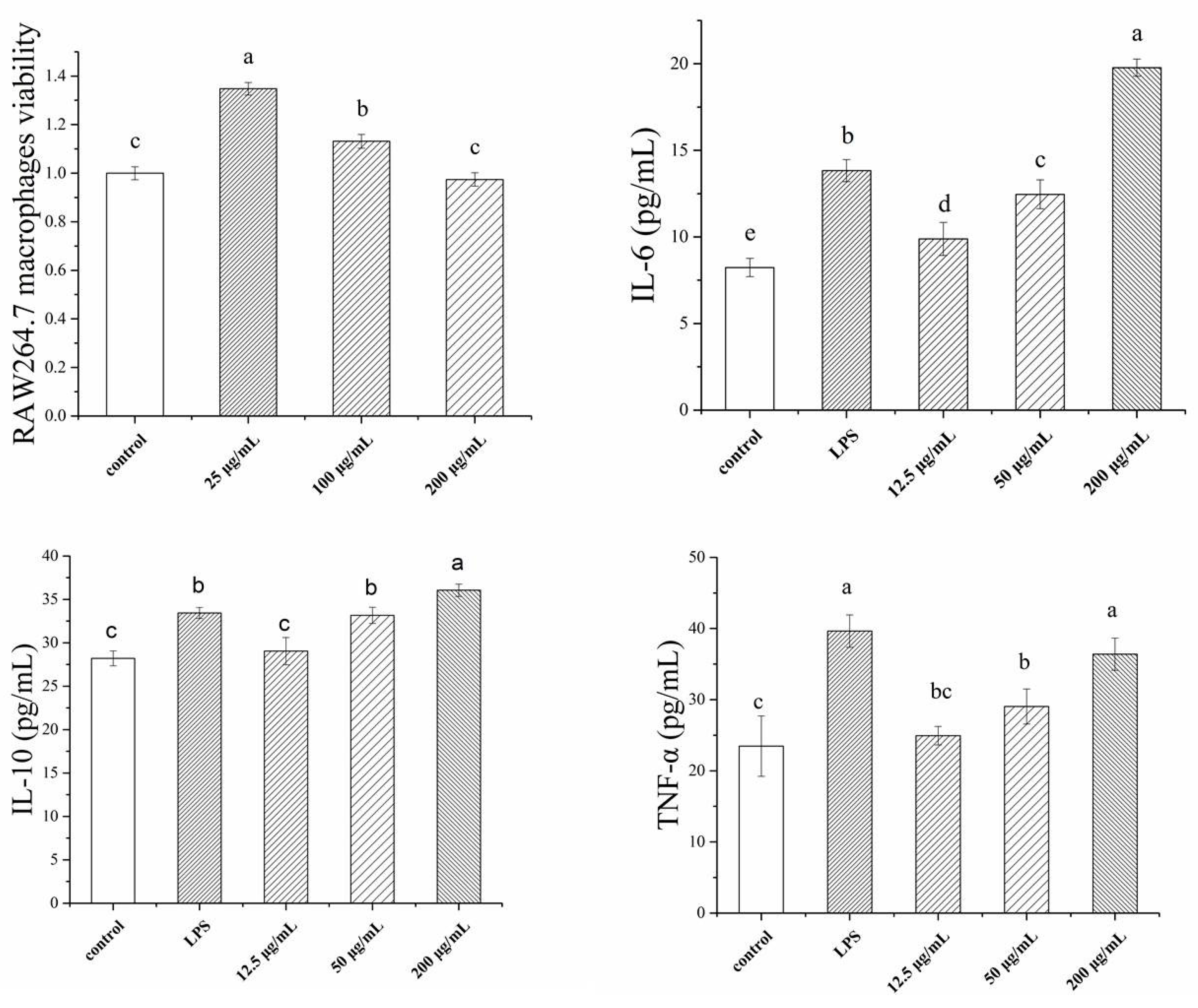
2.2. Immunological Activity of TSP
2.3. Anticancer Activity of TSP
2.3.1. Evaluating the Inhibition of HepG2 Growth Activity In Vitro
2.3.2. Cell Apoptosis Assessment Results
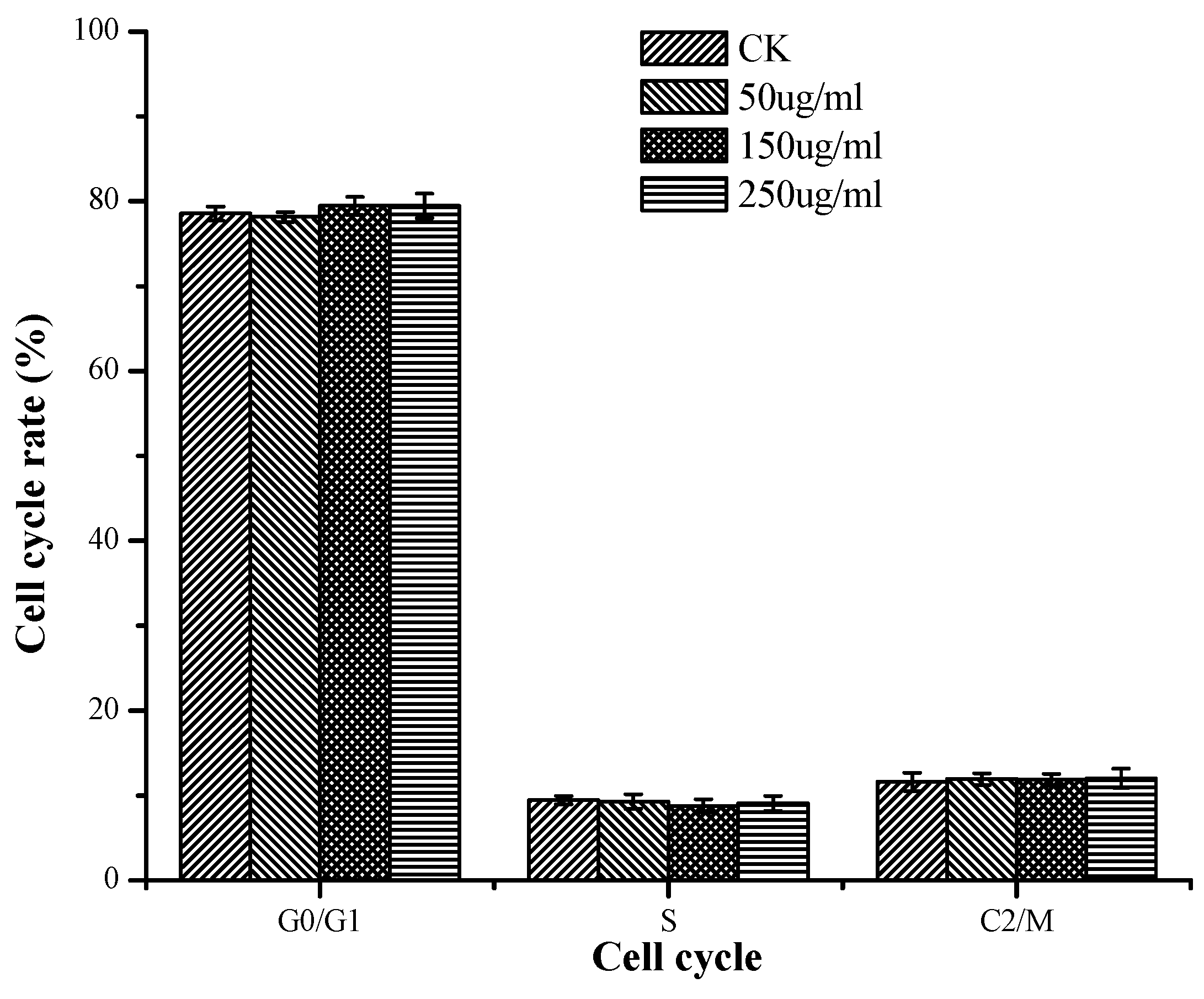
2.3.3. Induction of Apoptosis According to Cell Cycle Analysis
3. Discussion
4. Materials and Methods
4.1. Tribonema sp. Samples and Reagents
4.2. Extraction of Polysaccharides from Tribonema sp. (TSP)
4.3. Chemical Characterization
4.4. Immunological Activity Determination
4.5. Anticancer Activity of TSP
4.5.1. Cell Culture
4.5.2. Evaluation of Inhibiting HepG2 Growth Activity In Vitro
4.5.3. Apoptosis Assessment
4.5.4. Analysis of the Cell Cycle
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balat, M. Progress in biogas production processes. Energy Educ. Sci. Technol. 2008, 22, 15–36. [Google Scholar]
- Hamilton, M.L.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab. Eng. 2014, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.H.; Hu, D.X.; Balamurugan, S.; Lu, Y.; Yang, W.D.; Liu, J.S.; Li, H.Y. Identification of a putative patatin-like phospholipase domain-containing protein 3(PNPLA3) ortholog involved in lipid metabolism in microalga Phaeodactylum tricornutum. Algal Res. 2015, 12, 274–279. [Google Scholar] [CrossRef]
- Scott, S.A.; Davey, M.P.; Dennis, J.S.; Horst, I.; Howe, C.J.; Lea-Smith, D.J.; Smith, A.C. Biodiesel from algae: Challenges and prospects. Curr. Opin. Biotechnol. 2010, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, W.J.; Shao, H.M.; Liu, T.Z. A comparative analysis of biomass and lipid content in five Tribonema sp. strains at autotrophic, heterotrophic and mixotrophic cultivation. Algal Res. 2017, 24, 284–289. [Google Scholar] [CrossRef]
- Wang, H.; Ji, B.; Wang, J.F.; Guo, F.J.; Zhou, W.J.; Gao, L.L.; Liu, T.Z. Growth and biochemical composition of filamentous microalgae Tribonema sp. as potential biofuel feedstocks. Bioproc. Biosyst. Eng. 2014, 37, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, L.L.; Chen, L.; Guo, F.J.; Liu, T.Z. Integration process of biodiesel production from filamentous oleaginous microalgae Tribonema minus. Bioresour. Technol. 2013, 142, 39–44. [Google Scholar] [CrossRef]
- Sun, Y.H.; Chen, X.L.; Cheng, Z.Q.; Liu, S.; Yu, H.H.; Wang, X.Q.; Li, P.C. Degradation of polysaccharides from Grateloupia filicina and their antiviral activity to avian leucosis virus subgroup. J. Mar. Drugs 2017, 15, 345. [Google Scholar] [CrossRef]
- Kusaikin, M.I.; Ermakova, S.P.; Shevchenko, N.M.; Isakov, V.V.; Gorshkov, A.G.; Vereshchagin, A.L.; Grachev, M.A.; Zvyagintseva, T.N. Structural characteristics and antitumor activity of a new chrysolaminaran from the diatom alga Synedra acus. Chem. Nat. Compd. 2010, 46, 1–4. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.L.; Liu, X.D.; Zhang, F.B.; Hu, L.F.; Yue, Y.; Li, K.C.; Li, P.C. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar. Drugs 2016, 14, 4. [Google Scholar] [CrossRef]
- Huang, J.; Liu, L.P.; Yu, Y.; Lin, W.; Chen, B.L.; Li, M.; Rao, X.Z. Reduction in the blood glucose level of exopolysaccharide of Porhyridium cruentum in alloxan-induced diabetic mice. J. Fujian Normal Univ. 2006, 22, 77–80. [Google Scholar]
- Wijesinghe, W.A.J.P.; Athukorala, Y.; Jeon, Y.J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on wistar rat. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Xing, R.E.; Li, K.C.; Li, R.F.; Qin, Y.K.; Wang, X.Q.; Wei, Z.H.; Li, P.C. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013, 92, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Kurd, F.; Samavati, V. Water soluble polysaccharides from Spirulina platensis: Extraction and in vitro anti-cancer activity. Int. J. Biol. Macromol. 2015, 74, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Heaneykieras, J.; Chapman, D.J. Structure studies on extracellular polysaccharide of rea alga, Porphyridium cruentum. Carbohydr. Res. 1976, 52, 169–177. [Google Scholar]
- Kashif, S.A.; Hwang, Y.J.; Park, J.K. Potent biomedical applications of isolated polysaccharides from marine microalgae Tetraselmis species. Bioproc. Biosyst. Eng. 2018, 41, 1611–1620. [Google Scholar] [CrossRef]
- Orily, D.; Guillaume, P.; Wim, N.; Dries, V.; Imogen, F.; Philippe, M.; Koenraad, M. Influence of culture medium recycling on the performance of Arthrospira platensis cultures. Algal Res. 2015, 10, 48–54. [Google Scholar]
- Andrade, K.A.M.; Lauritano, C.; Romano, G.; Ianora, A. Marine microalgae with anti-cancer properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Senthilkumar, N.; Thangam, R.; Rajkumar, M.; Anbazhagan, C.; Rengasamy, R.; Gunasekaran, P.; Kannan, S.; Palani, P. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process Biochem. 2013, 48, 364–373. [Google Scholar] [CrossRef]
- Cédric, D.; Guillaume, P.; Céline, L.; Philippe, M. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar]
- Mourão, P.A.S. A carbohydrate-based mechanism of species recognition in sea urchin gertilization. Braz. J. Med. Biol. Res. 2007, 40, 5–17. [Google Scholar] [CrossRef]
- Nader, H.B.; Lopes, C.C.; Rocha, H.A.O.; Santos, E.A.; Dietrich, C.P. Heparins and heparinoids: Occurrence, structure and mechanism of antithrombotic and hemorrhagic activities. Curr. Pharm. Des. 2004, 10, 951–966. [Google Scholar] [CrossRef]
- Pérez-Recalde, M.; Matulewicz, M.C.; Pujol, C.A.; Carlucci, M.J. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from red seaweed Nemalion helminthoides. Int. J. Biol. Macromol. 2014, 63, 38–42. [Google Scholar] [CrossRef]
- Sousa, A.P.A.; Tores, M.R.; Pessoa, C.; Moraes, M.O.; Rocha Filho, F.D.; Alves, A.P.N.N.; Costa-Lotufo, L.V. In vivo growth-inhibition of sarcoma 180 tumor by alginates from brown seaweed Sargassum vulgare. Carbohydr. Polym. 2007, 69, 7–13. [Google Scholar] [CrossRef]
- Saito, A.; Yoneda, M.; Yokohama, S.; Okada, M.; Haneda, M.; Nakamura, K. Fucoidan prevents concanavalin A-induced liver injury through induction of endogenous IL-10 in mice. Hepatol. Res. 2006, 35, 190–198. [Google Scholar] [CrossRef] [Green Version]
- You, S.G.; Yang, C.; Lee, H.Y.; Lee, B.Y. Molecular characteristics of partially hydrolyzed fucoidans from sporophyll of Undaria pinnatifida and their in vitro anticancer activity. Food Chem. 2010, 119, 554–559. [Google Scholar] [CrossRef]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Tabarsa, M.; Cho, M.; You, S.G. Characterization and immunomodulatory activities of sulfated polysaccharides from Capsosiphon fulvescens. Int. J. Biol. Macromol. 2012, 51, 720–729. [Google Scholar] [CrossRef]
- Kawashima, T.; Murakami, K.; Nishimura, I.; Nakano, T.; Obata, A. A sulfated polysaccharide, fucoidan, enhances the immunomodulatory effects of lactic acid bacteria. Int. J. Mol. Med. 2012, 29, 447–453. [Google Scholar] [CrossRef]
- Maeda, R.; Ida, T.; Ihara, H.; Sakamoto, T. Immunostimulatory activity of polysaccharides isolated from Caulerpa lentillifera on macrophage cells. Biosci. Biotechnol. Biochem. 2012, 76, 501–505. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Chabrillón, M.; Cabello-pasini, A.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Characterization of polysaccharides from Hypnea spinella(Gigartinales) and Halopithys incurve(Ceramiales) and their effect on RAW 264.7 macrophage activity. J. Appl. Phycol. 2011, 23, 523–528. [Google Scholar] [CrossRef]
- Guzman, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophara and Phaeodactylum tricomutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Q.; Chu, J.L.; Sun, Z.L.; Chen, L.H. Physicochemical properties, immunomodulation and antitumor activities of polysaccharide from Pavlova viridis. Life Sci. 2016, 144, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Park, G.T.; Go, R.E.; Lee, H.M.; Lee, G.A.; Kim, C.W.; Seo, J.W.; Hong, W.K.; Choi, K.C.; Hwang, K.A. Potential anti-proliferative and immunomodulatory effects of marine microalgal exopolysaccharide on various human cancer cells and lymphocytes in vitro. Mar. Biotechnol. 2017, 19, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Pugh, N.D.; Ma, G.Y.; Pasco, D.S. Toll-like receptor 2-dependent activation of monocytes by spirulina polysaccharide and its immune enhancing action in mice. Int. Immunopharmacol. 2006, 6, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhang, S.H.; Yang, X.L.; Xu, H.B. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int. Immunopharmacol. 2004, 4, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Y.; Zhang, L.; Ge, W.; Tang, C.; Jia, Y.H.; Guo, C.S.; Wang, Q.J. Inhibitory effects of high dosage compound PSP on sarcoma 180 of mice. J. Liaoning Univ. Tradit. Chin. Med. 2008, 10, 179–181. [Google Scholar]
- Di, J.X.; Wang, J. Experimental study of Spirulina polysaccharides on the proliferation and apoptosis rate of hepatoma carcinoma cells induced by radiation. Western J. Tradit. Chin. Med. 2013, 26, 20–22. [Google Scholar]
- Xu, X.J.; Zhang, Y.; Tang, C.; Ge, W.; Liu, Y.J.; Wang, Q.J. Antitumor function of compound polysaccharides from Spirulina platensis on human HT-29 cell line in vitro. Lishizhen Med. Mater. Med. Res. 2012, 23, 2164–2166. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K.A. A modified method for chondrosulfatase assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Sung, N.Y.; Kim, H.M.; Byun, E.B.; Park, J.N.; Park, C.; Byun, M.W.; Byun, E.H. Polysaccharide extracted from Sargassum fulvellum leads to macrophage activation and Th1 polarization in splenocytes. Fish Sci. 2015, 81, 777–785. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from Xiaolin Chen. |
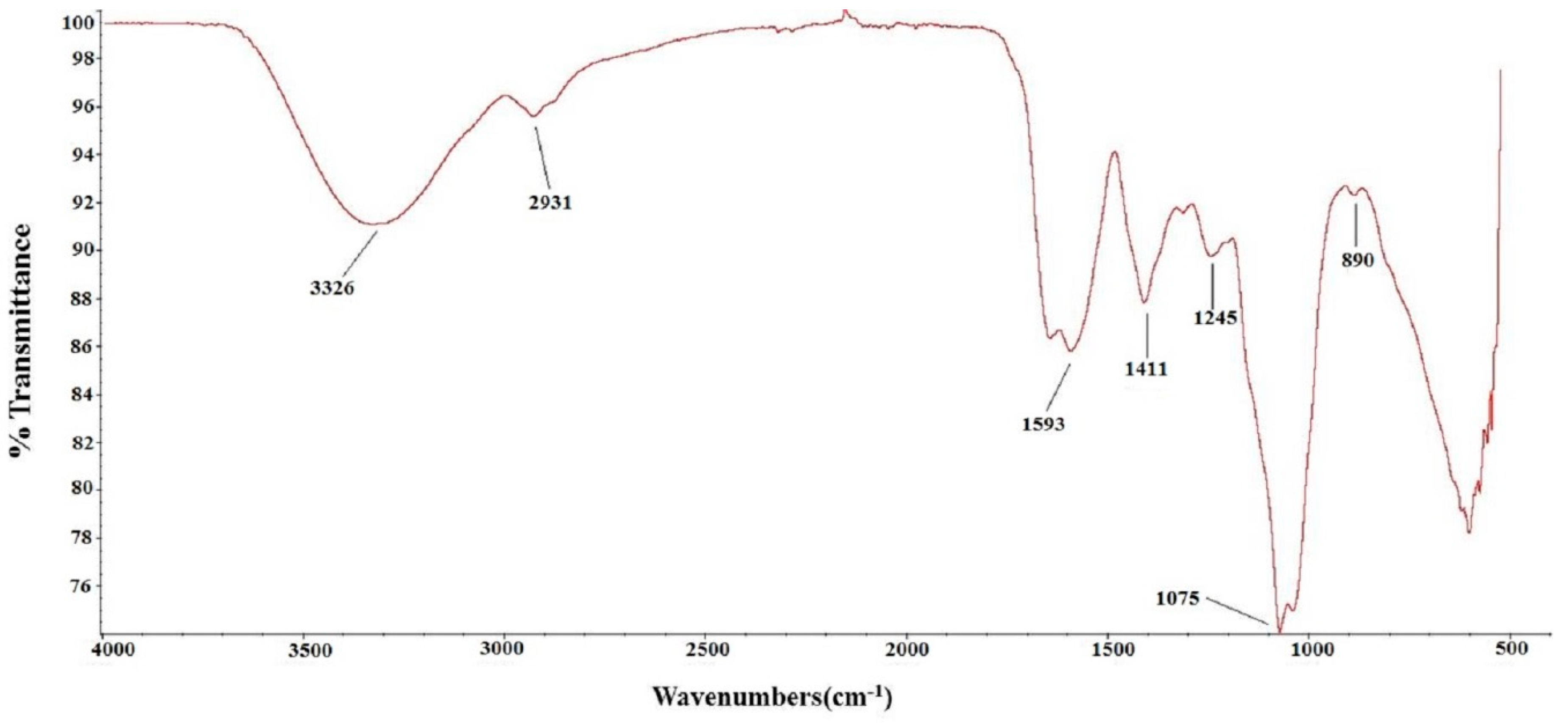


| Sample | Total Sugar/% | Sulfate/% | Mw/kDa | Monosaccharides Composition (Molar Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | Glc | Gal | Xyl | Fuc | ||||
| TSP | 32.5 ± 0.2 | 21.9 ± 0.15 | 197 ± 0.6 | 0.00 ± 0.00 | 0.101 ± 0.002 | 0.063 ± 0.001 | 0.069 ± 0.004 | 1.00 ± 0.03 | 0.32 ± 0.01 | 0.095 ± 0.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, R.; Liu, T.; Li, P. Partial Characterization, the Immune Modulation and Anticancer Activities of Sulfated Polysaccharides from Filamentous Microalgae Tribonema sp. Molecules 2019, 24, 322. https://doi.org/10.3390/molecules24020322
Chen X, Song L, Wang H, Liu S, Yu H, Wang X, Li R, Liu T, Li P. Partial Characterization, the Immune Modulation and Anticancer Activities of Sulfated Polysaccharides from Filamentous Microalgae Tribonema sp. Molecules. 2019; 24(2):322. https://doi.org/10.3390/molecules24020322
Chicago/Turabian StyleChen, Xiaolin, Lin Song, Hui Wang, Song Liu, Huahua Yu, Xueqin Wang, Rongfeng Li, Tianzhong Liu, and Pengcheng Li. 2019. "Partial Characterization, the Immune Modulation and Anticancer Activities of Sulfated Polysaccharides from Filamentous Microalgae Tribonema sp." Molecules 24, no. 2: 322. https://doi.org/10.3390/molecules24020322
APA StyleChen, X., Song, L., Wang, H., Liu, S., Yu, H., Wang, X., Li, R., Liu, T., & Li, P. (2019). Partial Characterization, the Immune Modulation and Anticancer Activities of Sulfated Polysaccharides from Filamentous Microalgae Tribonema sp. Molecules, 24(2), 322. https://doi.org/10.3390/molecules24020322








