Discrimination of Structural and Immunological Features of Polysaccharides from Persimmon Leaves at Different Maturity Stages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of the Physicochemical Properties of Three PLE0s
2.1.1. Chemical and Monosaccharide Compositions
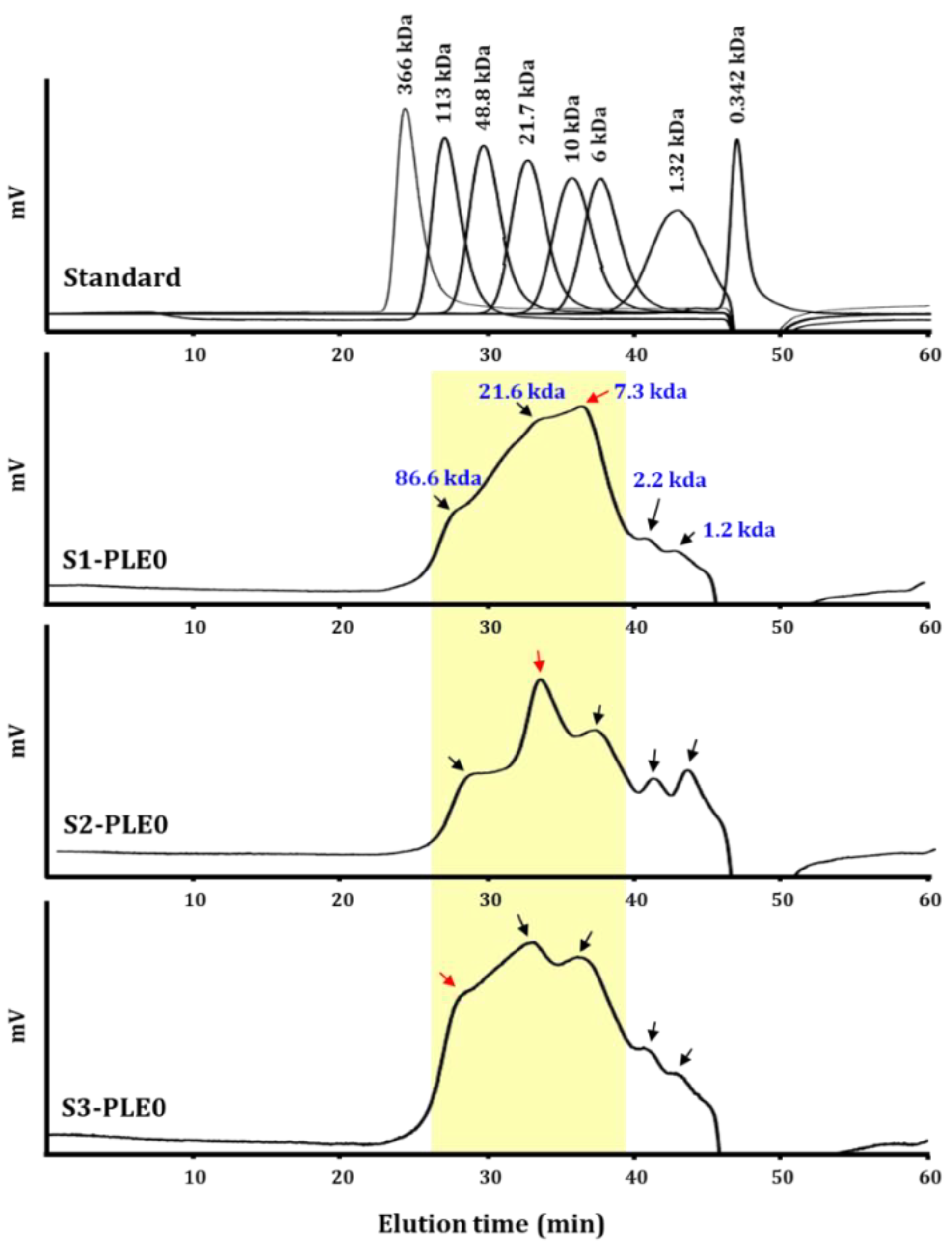
2.1.2. Molecular Weight Distribution
2.1.3. Linkage Analysis of Polysaccharides
2.2. Comparing the Immunostimulatory Activities of the Three PLE0s
2.2.1. Effects on Macrophage Viability and Phagocytic Ability
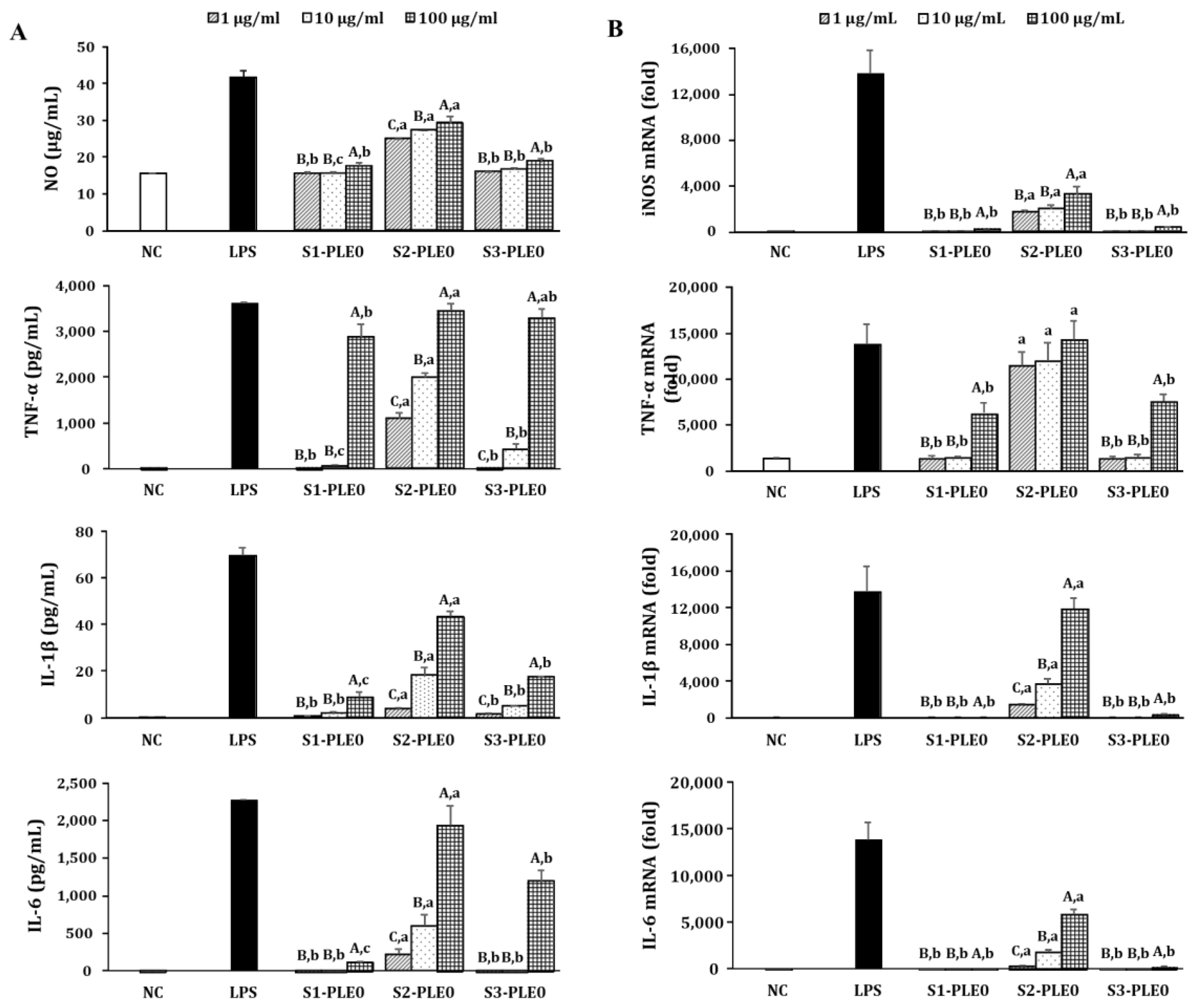
2.2.2. Effects on NO and Cytokine Production
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Polysaccharides from Persimmon Leaves at Different Maturity Stages
3.3. Chemical Component Analysis of Polysaccharides
3.4. Estimating the Molecular Weights of Polysaccharides
3.5. FT-IR Analysis
3.6. Immunostimulatory Activities of Polysaccharides
3.6.1. Cell Culture and Cytotoxicity Assays
3.6.2. Phagocytic Activity
3.6.3. Measurement of NO, TNF-α, IL-1β, and IL-6 Cytokine Secretion
3.6.4. qRT-PCR Analysis
3.6.5. Cytokine Array
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Dietary Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, X.; Xie, H.; Li, X.; Shi, L. Structural characterization and antitumor activity of a polysaccharide from Ramulus mori. Carbohydr. Polym. 2018, 190, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, X.B.; Zhao, J.W.; Lu, C.J.; Zhu, W. Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb. Carbohydr. Polym. 2017, 156, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Huang, H. Structural characterization of a novel polysaccharide fraction from Hericium erinaceus and its signaling pathways involved in macrophage immunomodulatory activity. J. Funct. Foods 2017, 37, 574–585. [Google Scholar] [CrossRef]
- Vannucci, L.; Krizan, J.; Sima, P.; Stakheev, D.; Caja, F.; Rajsiglova, L.; Horak, V.; Saieh, M. Immunostimulatory properties and antitumor activities of glucans. Int. J. Oncol. 2013, 43, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Burillo, S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2017, 105, 461–472. [Google Scholar] [CrossRef]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Kawakami, K.; Aketa, S.; Nakanami, M.; Iizuka, S.; Hirayama, M. Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their α-amylase inhibitory activity. Biosci. Biotechnol. Biochem. 2010, 74, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Li, F.; Xu, X.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2017, 94, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, Y.; Zhao, S.; Zhang, M.; Yan, X.; Gao, X.; Li, J.; Gao, Y.; Zhang, A.; Gao, Y. Antitumor and immunomodulatory activities of total flavonoids extract from persimmon leaves in H 22 liver tumor-bearing mice. Sci. Rep. 2018, 8, 10523. [Google Scholar] [CrossRef] [PubMed]
- Thuong, P.T.; Lee, C.H.; Dao, T.T.; Nguyen, P.H.; Kim, W.G.; Lee, S.J.; Oh, W.K. Triterpenoids from the leaves of Diospyros kaki (persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Shibukura, Y.; Kanno, T.; Furuki, T.; Aketa, S.; Hirayama, M. Identification of 2″-galloylated flavonol 3-O-glycosides accumulating in developing leaves of persimmon. Phytochem. Anal. 2011, 22, 403–410. [Google Scholar] [CrossRef]
- Duan, J.; Dong, Q.; Ding, K.; Fang, J. Characterization of a pectic polysaccharide from the leaves of Diospyros kaki and its modulating activity on lymphocyte proliferation. Biopolymers 2010, 93, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; He, M.; Li, J.; Luo, X.Y.; Huang, R. Hypoglycemic effect of persimmon leaf polysaccharide in diabetic mice induced by streptozotocin. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 114–117. [Google Scholar]
- Kim, H.; Hong, H.D.; Suh, H.J.; Shin, K.S. Structural and immunological feature of rhamnogalacturonan I-rich polysaccharide from Korean persimmon vinegar. Int. J. Biol. Macromol. 2016, 89, 319–327. [Google Scholar] [CrossRef]
- Park, H.R.; Hwang, D.; Hong, H.D.; Shin, K.S. Antitumor and antimetastatic activities of pectic polysaccharides isolated from persimmon leaves mediated by enhanced natural killer cell activity. J. Funct. Foods 2017, 37, 460–466. [Google Scholar] [CrossRef]
- Lee, S.G.; Jung, J.Y.; Shin, J.S.; Shin, K.S.; Cho, C.W.; Rhee, Y.K.; Hong, H.D.; Lee, K.T. Immunostimulatory polysaccharide isolated from the leaves of Diospyros kaki Thumb modulate macrophage via TLR2. Int. J. Biol. Macromol. 2015, 79, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Lee, H.; Hong, H.D.; Shin, K.S. Characterization of immunostimulatory pectic polysaccharide isolated from leaves of Diospyros kaki Thumb. (Persimmon). J. Funct. Foods 2016, 26, 319–329. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X. Optimization of extraction process of crude polysaccharides from pomegranate peel by response surface methodology. Carbohydr. Polym. 2013, 92, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, S.; Zhou, M.; Shang, X.; Yang, W.; Fu, X. Geographic variation in water-soluble polysaccharide content and antioxidant activities of Cyclocarya paliurus leaves. Ind. Crops Prod. 2018, 121, 180–186. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Ashkani, S.; Rahmat, A.; Juraimi, A.S.; Puteh, A.; Muda Mohamed, M.T. Variation in secondary metabolite production as well as antioxidant and antibacterial activities of Zingiber zerumbet (L.) at different stages of growth. BMC Complement. Altern. Med. 2016, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.B.; Hong, Y.S.; Lee, G.H.; Park, Y.M.; Lee, C.M.; Nho, E.Y.; Choi, J.Y.; Jamila, N.; Khan, N.; Kim, K.S. Determination of volatile organic compounds, catechins, caffeine and theanine in Jukro tea at three growth stages by chromatographic and spectrometric methods. Food Chem. 2017, 219, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.Y.; Jeong, J.M. Change of antioxidative activity at different harvest time and improvement of atopic dermatitis effects for persimmon leaf extract. Korea J. Herbol. 2012, 27, 41–49. [Google Scholar] [CrossRef]
- Patova, O.A.; Golovchenko, V.V.; Ovodov, Y.S. Pectic polysaccharides: Structure and properties. Russ. Chem. Bull. 2014, 63, 1901–1924. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Isolation, purification and physicochemical properties of polysaccharide from fruiting body of Hericium erinaceus and its effect on colonic health of mice. Int. J. Biol. Macromol. 2018, 107, 1310–1319. [Google Scholar] [CrossRef]
- Ognyanov, M.; Georgiev, Y.; Petkova, N.; Ivanov, I.; Vasileva, I.; Kratchanova, M. Isolation and characterization of pectic polysaccharide fraction from in vitro suspension culture of Fumaria officinalis L. Int. J. Polym. Sci. 2018, 5705036. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, S.; Zhu, P.; Nie, C.; Ma, S.; Wang, N.; Du, X.; Zhou, Y. Purification, characterization and immunomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2018, 107, 882–890. [Google Scholar] [CrossRef]
- Sun, Y.; Gong, G.; Guo, Y.; Wang, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J. Purification, structural features and immunostimulatory activity of novel polysaccharides from Caulerpa lentillifera. Int. J. Biol. Macromol. 2018, 108, 314–323. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Mamura, M.; Barlic-Dicen, J.; Grage-Griebenow, E. Pathophysiological roles of cytokine-chemokine immune network. J. Immunol. Res. 2014, 2014, 615130. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Samples | S1-PLE0 | S2-PLE0 | S3-PLE0 |
|---|---|---|---|
| Yield (%) 1 | 1.80 ± 0.23 a | 1.44 ± 0.18 ab | 1.20 ± 0.24 b |
| Chemical composition (%) | |||
| Neutral sugar | 65.3 ± 3.6 a | 57.0 ± 2.7 b | 62.5 ± 3.9 ab |
| Uronic acid | 32.9 ± 3.4 b | 40.9 ± 3.4 a | 35.2 ± 2.6 ab |
| Protein | 0.6 ± 0.1 b | 0.8 ± 0.0 ab | 0.9 ± 0.1 a |
| KDO 2-like material | 1.2 ± 0.1 | 1.4 ± 0.3 | 1.3 ± 0.1 |
| Sugar composition (molar ratio) | |||
| Galactose | 2.06 | 4.46 | 4.07 |
| Arabinose | 0.60 | 1.59 | 0.94 |
| Glucose | 1.00 | 1.00 | 1.00 |
| Rhamnose | 0.31 | 0.75 | 0.11 |
| Mannose | 0.20 | 0.35 | 0.41 |
| Xylose | 0.16 | 0.30 | 0.09 |
| Fucose | 0.03 | 0.09 | 0.04 |
| Galacturonic acid | 7.78 | 10.94 | 3.95 |
| Glucuronic acid | 0.24 | 0.43 | 0.45 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| iNOS | 5′-CCAGCCTGCCCCTTCAAT-3′ | 5′-ATCCTTCGGCCCACCTTCCT-3′ |
| TNF-α | 5′-AGGCACTCCCCCAAAAGATG-3′ | 5′-CACCCCGAAGTTCAGTAGACAGA-3′ |
| IL-1β | 5′-TGACGGACCCCAAAAGAT-3′ | 5′-GTGATACTGCCTGCCTGAAG-3′ |
| IL-6 | 5′-CCGGAGAGGAGACTTCACAGAG-3′ | 5′-TCATTTCCACGATTTCCCAGAG-3′ |
| GAPDH | 5′-CATGGCCTTCCGTGTTCCTAC-3′ | 5′-TCAGTGGGCCCTCAGATGC-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-R.; Han, A.-R.; Lim, T.-G.; Kang, J.-H.; Hong, H.-D. Discrimination of Structural and Immunological Features of Polysaccharides from Persimmon Leaves at Different Maturity Stages. Molecules 2019, 24, 356. https://doi.org/10.3390/molecules24020356
Song Y-R, Han A-R, Lim T-G, Kang J-H, Hong H-D. Discrimination of Structural and Immunological Features of Polysaccharides from Persimmon Leaves at Different Maturity Stages. Molecules. 2019; 24(2):356. https://doi.org/10.3390/molecules24020356
Chicago/Turabian StyleSong, Young-Ran, Ah-Ram Han, Tae-Gyu Lim, Ji-Hyun Kang, and Hee-Do Hong. 2019. "Discrimination of Structural and Immunological Features of Polysaccharides from Persimmon Leaves at Different Maturity Stages" Molecules 24, no. 2: 356. https://doi.org/10.3390/molecules24020356
APA StyleSong, Y.-R., Han, A.-R., Lim, T.-G., Kang, J.-H., & Hong, H.-D. (2019). Discrimination of Structural and Immunological Features of Polysaccharides from Persimmon Leaves at Different Maturity Stages. Molecules, 24(2), 356. https://doi.org/10.3390/molecules24020356






