20(S)-Protopanaxadiol Saponins Mainly Contribute to the Anti-Atherogenic Effects of Panax notoginseng in ApoE Deficient Mice
Abstract
:1. Introduction
2. Results
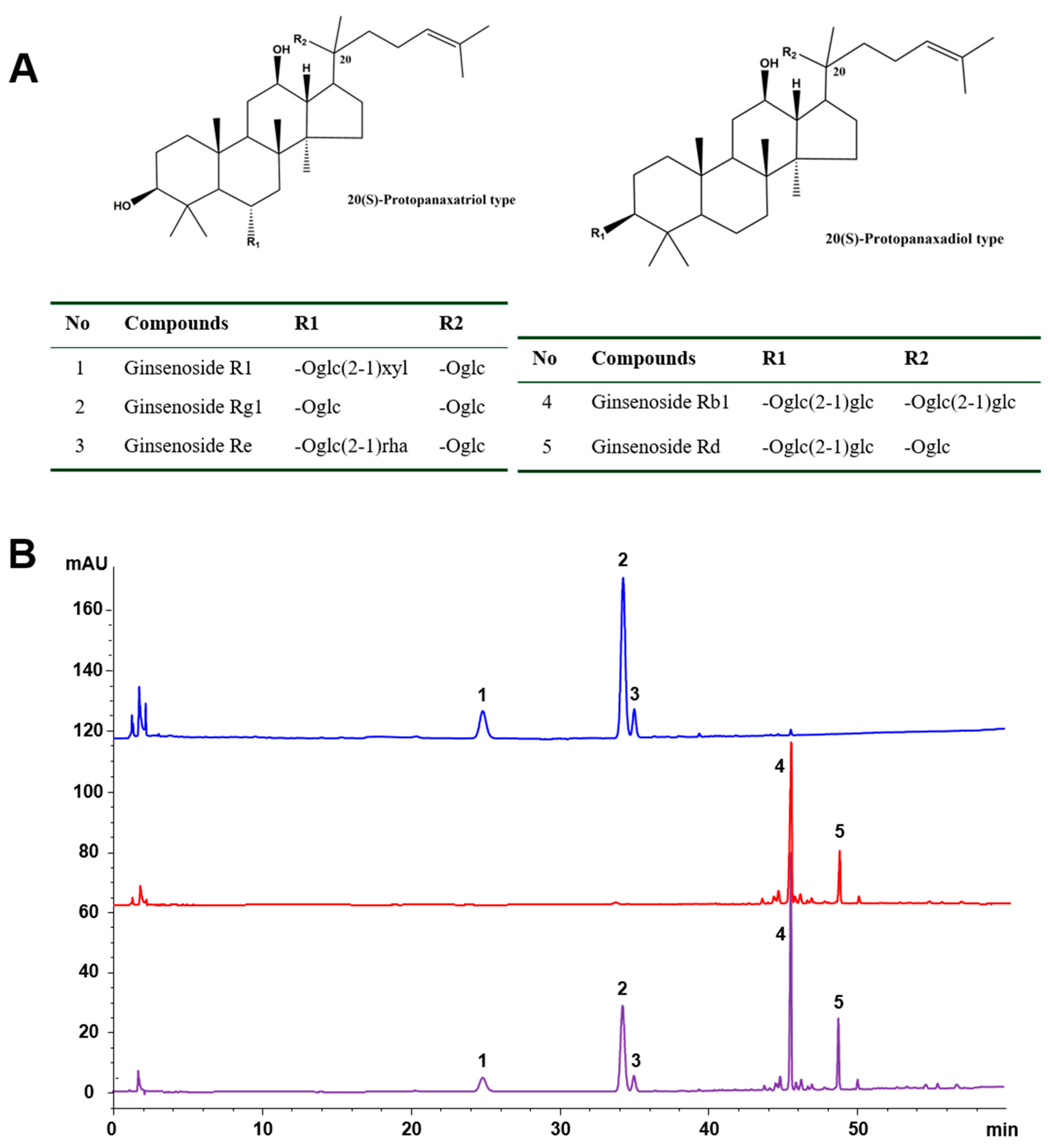
2.1. Chemical Characteristics of the Tested Samples
2.2. Effects of PNS, PDS, and PTS on Atherosclerotic Lesions
2.3. Levels of Plasma Lipids
2.4. Effects of PNS, PDS, and PTS on Plaque Vulnerability
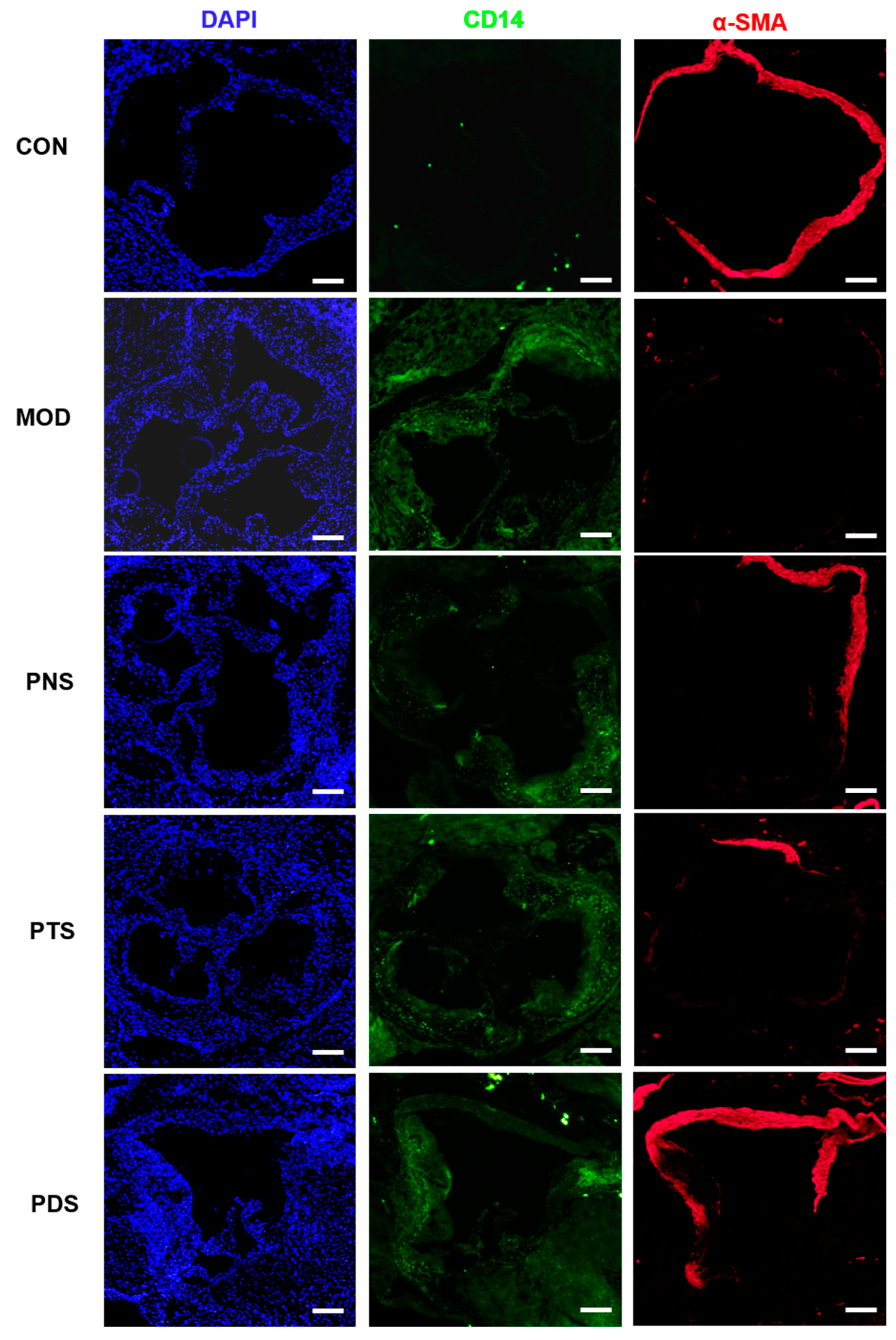
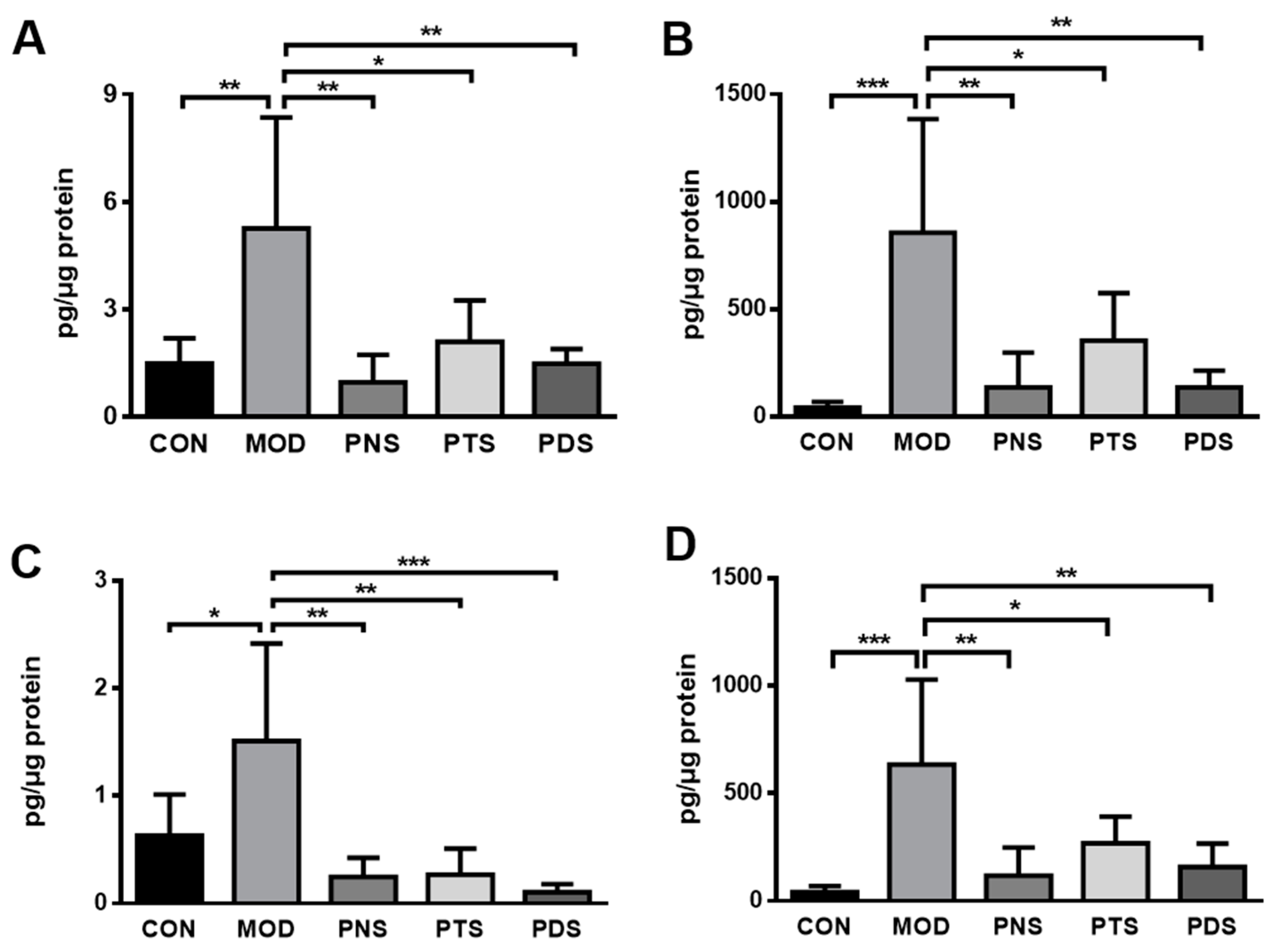
2.5. Effects of PNS, PDS, and PTS on Vascular Inflammation
3. Discussion
4. Materials and Methods
4.1. HPLC-UV Analysis of PNS, PTS, and PDS
4.2. Animals and Treatment
4.3. Measurement of Atherosclerotic Lesions
4.4. Plasma Lipid Analysis
4.5. Ex Vivo Aorta Tissue Culture and Cytokine Assays
4.6. Immunostaining Assay
4.7. qPCR Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Organization, W.H. Cardiovascular diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 25 August 2019).
- Badimon, L.; Vilahur, G.; Padro, T. Lipoproteins, platelets and atherothrombosis. Rev. Esp. Cardiol. 2009, 62, 1161–1178. [Google Scholar] [CrossRef]
- Wan, J.B.; Lee, S.M.; Wang, J.D.; Wang, N.; He, C.W.; Wang, Y.T.; Kang, J.X. Panax notoginseng reduces atherosclerotic lesions in ApoE-deficient mice and inhibits TNF-alpha-induced endothelial adhesion molecule expression and monocyte adhesion. J. Agric. Food Chem. 2009, 57, 6692–6697. [Google Scholar] [CrossRef] [PubMed]
- Weissberg, P.L.; Bennett, M.R. Atherosclerosis - an inflammatory disease. N. Engl. J. Med. 1999, 340, 1928–1929. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscle. Throm. Vas. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-J.; Su, H.; Wang, Y.-T.; Wan, J.-B. Therapeutic Potential of Ginsenosides in Management of Atherosclerosis. In Phytotherapies: Efficacy, Safety, and Regulation; Ramzan, I., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Liu, G.; Wang, B.; Zhang, J.; Jiang, H.; Liu, F. Total panax notoginsenosides prevent atherosclerosis in apolipoprotein E-knockout mice: Role of downregulation of CD40 and MMP-9 expression. J. Ethnopharmacol. 2009, 126, 350–354. [Google Scholar] [CrossRef]
- Dou, L.; Lu, Y.; Shen, T.; Huang, X.; Man, Y.; Wang, S.; Li, J. Panax notogingseng saponins suppress RAGE/MAPK signaling and NF-kappaB activation in apolipoprotein-E-deficient atherosclerosis-prone mice. Cell Physiol. Biochem. 2012, 29, 875–882. [Google Scholar] [CrossRef]
- Kim, C.K.; Cho, D.H.; Lee, K.S.; Lee, D.K.; Park, C.W.; Kim, W.G.; Lee, S.J.; Ha, K.S.; Goo Taeg, O.; Kwon, Y.G.; et al. Ginseng Berry Extract Prevents Atherogenesis via Anti-Inflammatory Action by Upregulating Phase II Gene Expression. Evid. Based Complement Alternat. Med. 2012, 2012, 490301. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.Z.; Tang, Y.B.; Zhou, J.G.; Guan, Y.Y. Ginsenoside-Rd, a purified component from panax notoginseng saponins, prevents atherosclerosis in apoE knockout mice. Eur. J. Pharmacol. 2011, 652, 104–110. [Google Scholar] [CrossRef]
- Fan, J.S.; Liu, D.N.; Huang, G.; Xu, Z.Z.; Jia, Y.; Zhang, H.G.; Li, X.H.; He, F.T. Panax notoginseng saponins attenuate atherosclerosis via reciprocal regulation of lipid metabolism and inflammation by inducing liver X receptor alpha expression. J. Ethnopharmacol. 2012, 142, 732–738. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhang, H.G.; Zhang, G.Y.; Fan, J.S.; Li, X.H.; Liu, Y.H.; Li, S.H.; Lian, X.M.; Tang, Z. Panax notoginseng saponins attenuate atherosclerosis in rats by regulating the blood lipid profile and an anti-inflammatory action. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1238–1244. [Google Scholar] [CrossRef]
- Yuan, Z.; Liao, Y.; Tian, G.; Li, H.; Jia, Y.; Zhang, H.; Tan, Z.; Li, X.; Deng, W.; Liu, K.; et al. Panax notoginseng saponins inhibit Zymosan A induced atherosclerosis by suppressing integrin expression, FAK activation and NF-kappaB translocation. J. Ethnopharmacol. 2011, 138, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.G.; Jia, Y.; Li, X.H. Panax notoginseng saponins attenuate atherogenesis accelerated by zymosan in rabbits. Biol. Pharm. Bull. 2010, 33, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Toh, S.A.; Sellers, L.A.; Skepper, J.N.; Koolwijk, P.; Leung, H.W.; Yeung, H.W.; Wong, R.N.; Sasisekharan, R.; Fan, T.P. Modulating angiogenesis: The yin and the yang in ginseng. Circulation 2004, 110, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Kong, L.D.; Ye, W.C.; Cheng, C.H.; Tan, R.X. Inhibition of xanthine and monoamine oxidases by stilbenoids from Veratrum taliense. Planta. Med. 2001, 67, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Matsumoto, N.; Zou, K.; Meselhy, M.R.; Komatsu, K. Axonal and dendritic extension by protopanaxadiol-type saponins from ginseng drugs in SK-N-SH cells. Jpn. J. Pharmacol. 2002, 90, 254–262. [Google Scholar] [CrossRef]
- Wang, N.; Wan, J.B.; Chan, S.W.; Deng, Y.H.; Yu, N.; Zhang, Q.W.; Wang, Y.T.; Lee, S.M.Y. Comparative study on saponin fractions from Panax notoginseng inhibiting inflammation-induced endothelial adhesion molecule expression and monocyte adhesion. Chin. Med. 2011, 6. [Google Scholar] [CrossRef]
- Wan, J.B.; Zhang, Q.W.; Ye, W.C.; Wang, Y.T. Quantification and separation of protopanaxatriol and protopanaxadiol type saponins from Panax notoginseng with macroporous resins. Sep. Purif. Technol. 2008, 60, 198–205. [Google Scholar] [CrossRef]
- Wan, J.B.; Lai, C.M.; Li, S.P.; Lee, M.Y.; Kong, L.Y.; Wang, Y.T. Simultaneous determination of nine saponins from Panax notoginseng using HPLC and pressurized liquid extraction. J. Pharmaceut. Biomed. 2006, 41, 274–279. [Google Scholar] [CrossRef]
- de Winther, M.P.; Kanters, E.; Kraal, G.; Hofker, M.H. Nuclear factor kappaB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 904–914. [Google Scholar] [CrossRef]
- Zhang, X.J.; He, C.; Tian, K.; Li, P.; Su, H.; Wan, J.B. Ginsenoside Rb1 attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of the JNK and p38 signaling pathways. Vascul. Pharmacol. 2015, 73, 86–95. [Google Scholar] [CrossRef]
- Yuan, C.S.; Wang, C.Z.; Wicks, S.M.; Qi, L.W. Chemical and pharmacological studies of saponins with a focus on American ginseng. J. Ginseng Res. 2010, 34, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.M.; Hsu, C.H.; Rajasekaran, S. Angiogenic evaluation of ginsenoside Rg 1 from Panax ginseng in fluorescent transgenic mice. Vascul. Pharmacol. 2008, 49, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.; Chen, S.C.; Chang, W.C.; Huang, Y.C.; Lin, K.M.; Lai, P.H.; Sung, H.W. Stability of angiogenic agents, ginsenoside Rg1 and Re, isolated from Panax ginseng: In vitro and in vivo studies. Int. J. Pharm. 2007, 328, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.W.; Ryu, J.H.; Oh, H.J. The influence of Sam-Chil-Geun (Panax notoginseng) on the serum lipid levels and inflammations of rats with hyperlipidemia induced by poloxamer-407. Yonsei Med. J. 2010, 51, 504–510. [Google Scholar] [CrossRef]
- Jiang, Q.F.; Huang, M.Y.; Wu, K.Y.; Weng, J.L.; Deng, R.G.; Xu, X.J.; Xu, J.P.; Jiang, T. Intervention Effects of Atorvastatin Combined with Panax notoginseng Saponins on Rats with Atherosclerosis Complicated with Hepatic Injury. Pharmacogn. Mag. 2017, 13, 430–438. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; de Winther, M.P.; Weber, C.; Daemen, M.J.; Lutgens, E.; Soehnlein, O. Atherosclerotic plaque destabilization: Mechanisms, models, and therapeutic strategies. Circ. Res. 2014, 114, 214–226. [Google Scholar] [CrossRef]
- Shiomi, M.; Ito, T.; Hirouchi, Y.; Enomoto, M. Fibromuscular cap composition is important for the stability of established atherosclerotic plaques in mature WHHL rabbits treated with statins. Atherosclerosis 2001, 157, 75–84. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]
- Fan, J.; Liu, D.; He, C.; Li, X.; He, F. Inhibiting adhesion events by Panax notoginseng saponins and Ginsenoside Rb1 protecting arteries via activation of Nrf2 and suppression of p38 - VCAM-1 signal pathway. J. Ethnopharmacol. 2016, 192, 423–430. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Luo, Y.; Lu, S.; Dai, Z.; Wang, R.; Zhang, X.; Li, G.; Sun, G.; Sun, X. Inhibitory Effects of Ginsenoside Rb1 on Early Atherosclerosis in ApoE-/- Mice via Inhibition of Apoptosis and Enhancing Autophagy. Molecules 2018, 23, 2912. [Google Scholar] [CrossRef]
- Jia, C.; Xiong, M.; Wang, P.; Cui, J.; Du, X.; Yang, Q.; Wang, W.; Chen, Y.; Zhang, T. Notoginsenoside R1 attenuates atherosclerotic lesions in ApoE deficient mouse model. PLoS ONE 2014, 9, e99849. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.B.; Li, P.; Yang, R.L.; Zhang, Q.W.; Wang, Y.T. Separation and purification of 5 saponins from panax notoginseng by preparative high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 406–417. [Google Scholar] [CrossRef]
- Wan, J.B.; Huang, L.L.; Rong, R.; Tan, R.; Wang, J.; Kang, J.X. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.B.; Chen, J.H.; Liu, C.H.; Xia, F.B.; Xiao, Z.Y.; Zhang, X.; Wan, J.B. A combination of Pueraria lobata and Silybum marianum protects against alcoholic liver disease in mice. Phytomedicine 2019, 58. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds, notoginsenoside R1, ginsenosides Rg1,Re, Rb1 and Rd are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Feng, R.; Zou, J.; Xia, F.; Wan, J.-B. 20(S)-Protopanaxadiol Saponins Mainly Contribute to the Anti-Atherogenic Effects of Panax notoginseng in ApoE Deficient Mice. Molecules 2019, 24, 3723. https://doi.org/10.3390/molecules24203723
Liu C, Feng R, Zou J, Xia F, Wan J-B. 20(S)-Protopanaxadiol Saponins Mainly Contribute to the Anti-Atherogenic Effects of Panax notoginseng in ApoE Deficient Mice. Molecules. 2019; 24(20):3723. https://doi.org/10.3390/molecules24203723
Chicago/Turabian StyleLiu, Conghui, Ruibing Feng, Jian Zou, Fangbo Xia, and Jian-Bo Wan. 2019. "20(S)-Protopanaxadiol Saponins Mainly Contribute to the Anti-Atherogenic Effects of Panax notoginseng in ApoE Deficient Mice" Molecules 24, no. 20: 3723. https://doi.org/10.3390/molecules24203723





