Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena versicolor Spider Molt Cuticle
Abstract
:1. Introduction
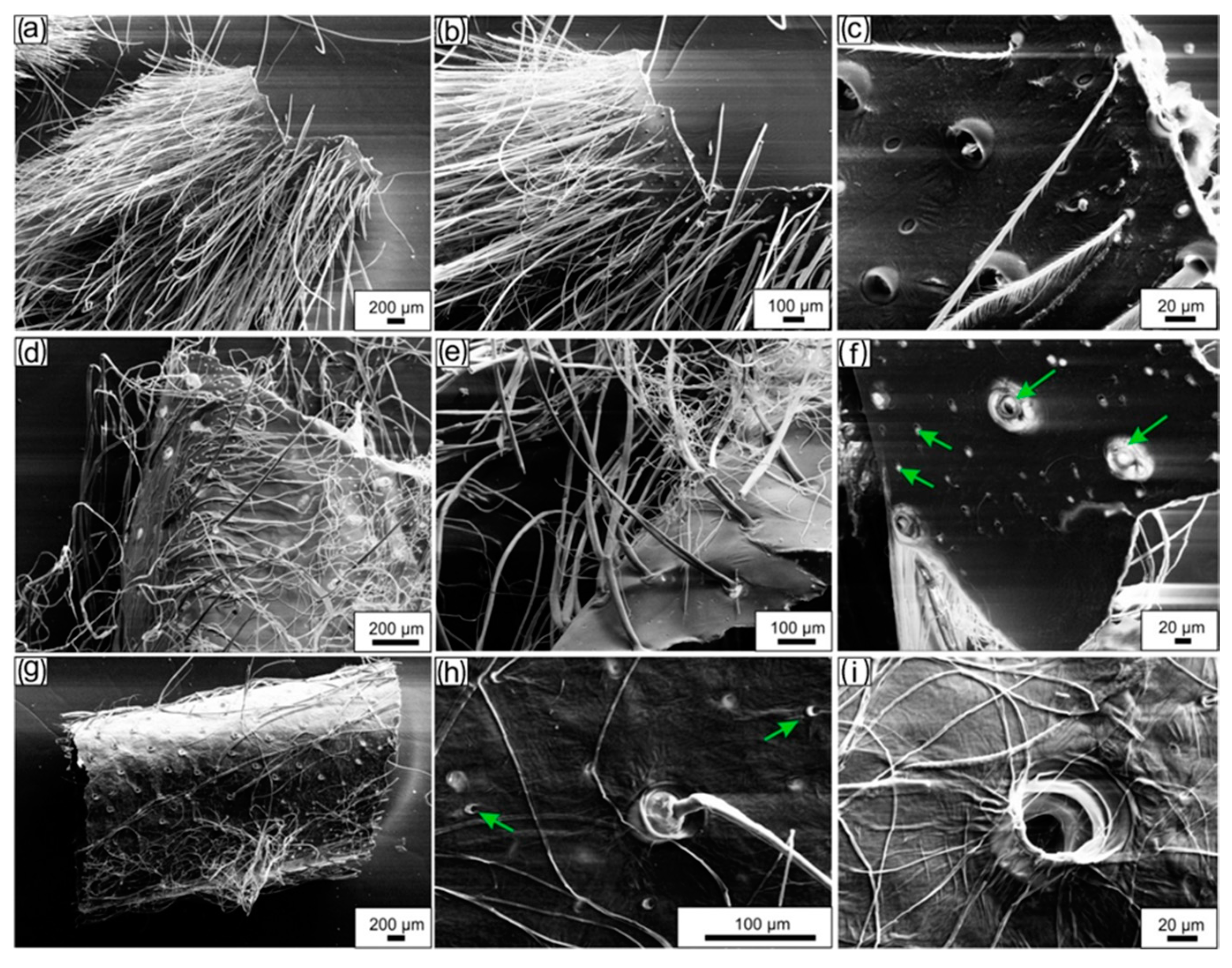
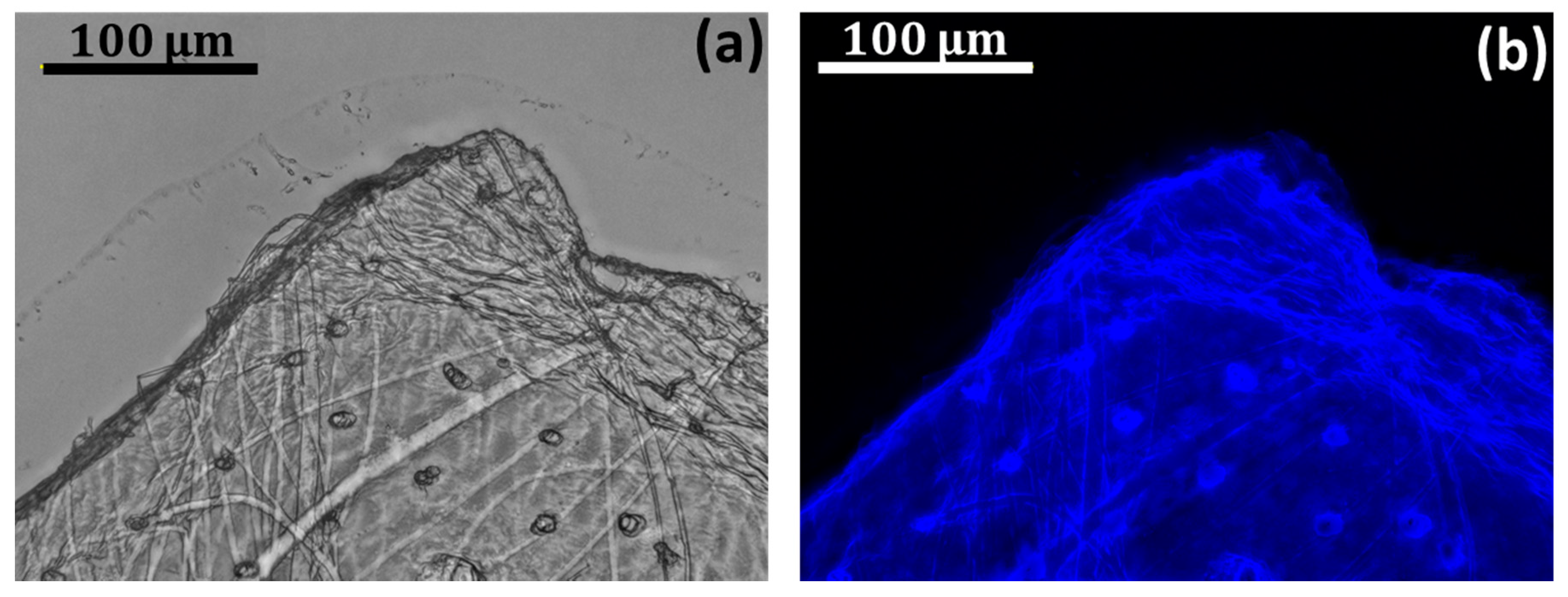
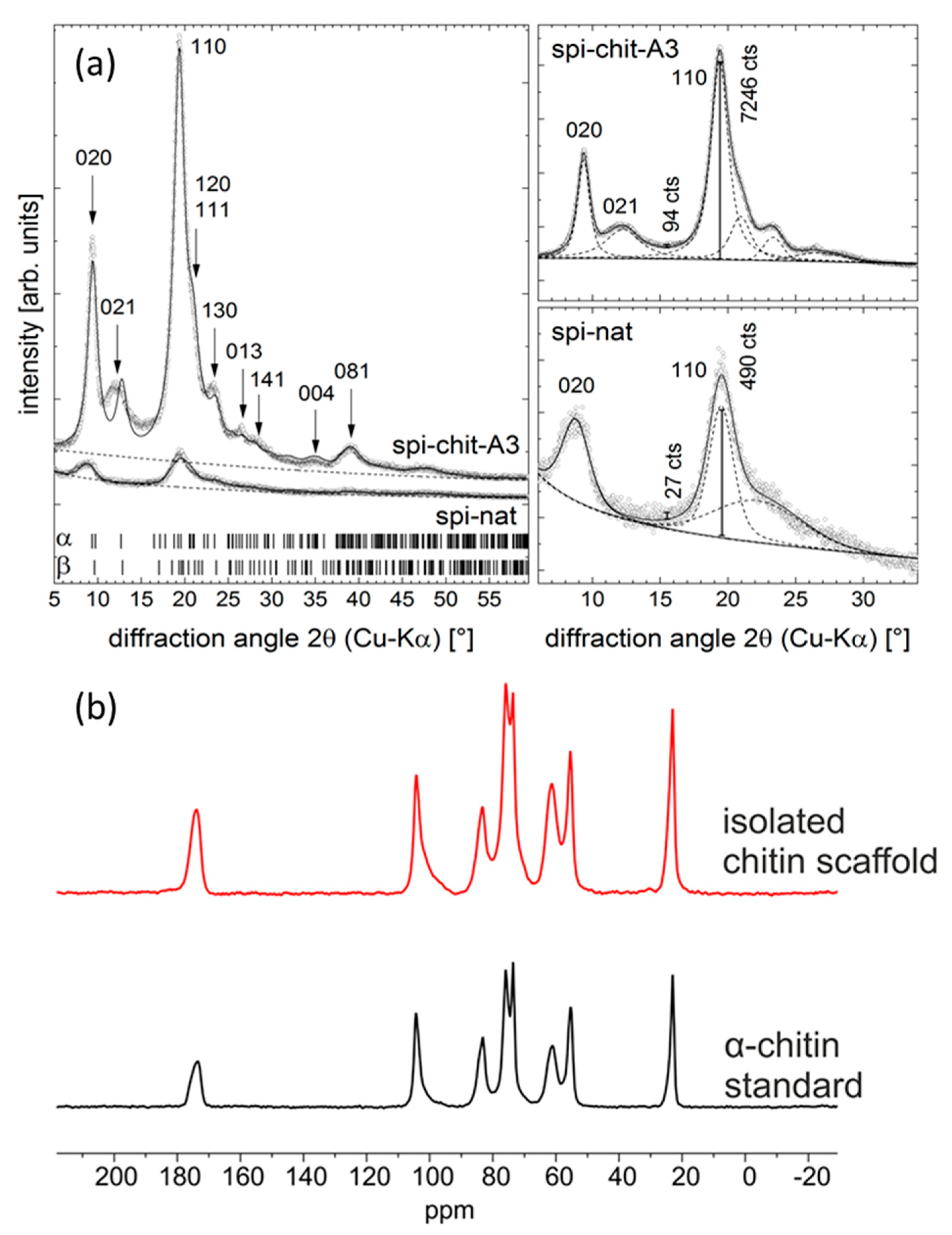
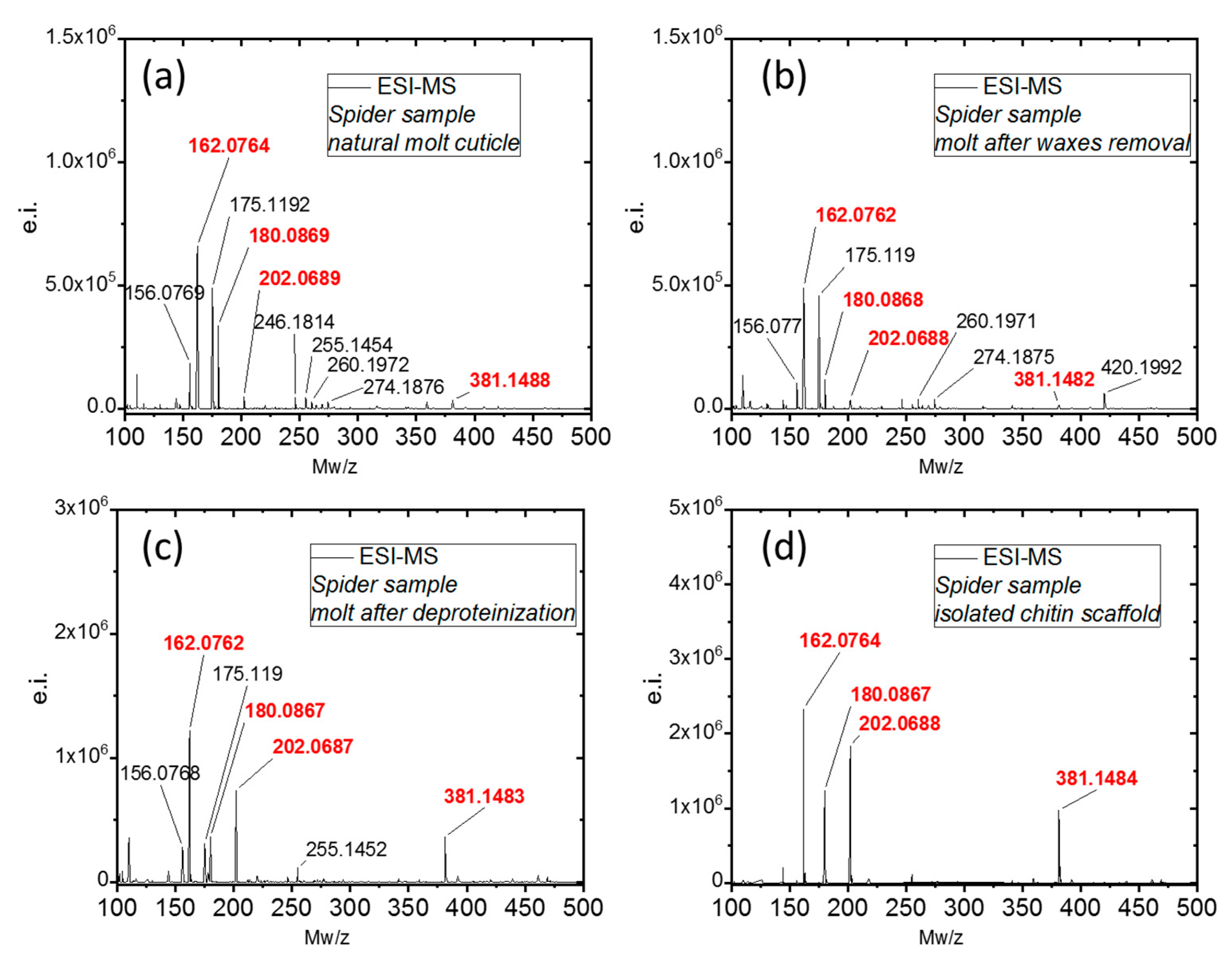
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Chitin Extraction
3.2.1. Microwave-Assisted Chitin Isolation
3.2.2. Estimation of Chitin Content in Molt
3.2.3. Pigment Isolation
3.3. Characterization of Obtained Materials
3.3.1. Light, Fluorescence, and Stereo Microscopy
3.3.2. Calcofluor White Staining
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
3.3.5. Raman Spectroscopy
3.3.6. X-ray Diffraction (XRD)
3.3.7. Electrospray Ionization Mass Spectrometry (ESI-MS)
3.3.8. 13C Solid State NMR
3.3.9. Chitinase Digestion Test
3.3.10. UV-VIS Spectroscopy
3.3.11. Parameters of the Porous Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bo, M.; Bavestrello, G.; Kurek, D.; Paasch, S.; Brunner, E.; Born, R.; Galli, R.; Stelling, A.L.; Sivkov, V.N.; Petrova, O.V.; et al. Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa: Cnidaria). Int. J. Biol. Macromol. 2012, 51, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; Van Pée, K.H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom thalassiosira pseudonana. Angew. Chemie Int. Ed. 2009, 48, 9724–9727. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Malando, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J. Exp. Zool. 2007, 308B, 347–357. [Google Scholar] [CrossRef]
- Erko, M.; Hartmann, M.A.; Zlotnikov, I.; Valverde Serrano, C.; Fratzl, P.; Politi, Y. Structural and mechanical properties of the arthropod cuticle: Comparison between the fang of the spider Cupiennius salei and the carapace of American lobster Homarus americanus. J. Struct. Biol. 2013, 183, 172–179. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Rahman, H.A.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum compactum. Sci. Rep. 2014, 22, 6162. [Google Scholar] [CrossRef]
- Rudall, K.M.; Kenchington, W. The chitin system. Biol. Rev. 1973, 48, 597–636. [Google Scholar] [CrossRef]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef]
- Wysokowski, M.; Zatoń, M.; Bazhenov, V.V.; Behm, T.; Ehrlich, A.; Stelling, A.L.; Hog, M.; Ehrlich, H. Identification of chitin in 200-million-year-old gastropod egg capsules. Paleobiology 2014, 40, 529–540. [Google Scholar] [CrossRef]
- Tang, W.J.; Fernandez, J.G.; Sohn, J.J.; Amemiya, C.T.; Tang, W.J.; Fernandez, J.G.; Sohn, J.J.; Amemiya, C.T. Chitin is endogenously produced in vertebrates. Curr. Biol. 2015, 25, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef]
- Zhang, X.; Rolandi, M. Engineering strategies for chitin nanofibers. J. Mater. Chem. B 2017, 5, 2547–2559. [Google Scholar] [CrossRef]
- Di Mario, F.; Rapana, P.; Tomati, U.; Galli, E. Chitin and chitosan from basidiomycetes. Int. J. Biol. Macromol. 2008, 43, 8–12. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Nawawi, W.M.F.W.; Lee, K.Y.; Kontturi, E.; Murphy, R.J.; Bismarck, A. Chitin nanopaper from mushroom extract: Natural composite of nanofibers and glucan from a single biobased source. ACS Sustain. Chem. Eng. 2019, 77, 6492–6496. [Google Scholar] [CrossRef]
- van Leeuwen, J.; Norton, G.A.; Ndlela, S.S.; Rudnick, D. Processes for Isolating Chitin and Chitosan from Fungal Biomass. U.S. Patents 9,249,235 B2, 2 February 2016. [Google Scholar]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Conway, W.S.; Sams, C.E. Physicochemical properties and bioactivity of fungal chitin and chitosan. J. Agric. Food Chem. 2005, 53, 3888–3894. [Google Scholar] [CrossRef]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overview. Biomacromolecules 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.S.; Khor, E. Novel fabrication of open-pore chitin matrixes. Biomacromolecules 2000, 1, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Asfour, H.Z.; Youssef, D.T.A.; Zółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K.; et al. New source of 3D chitin scaffolds: The Red Sea Demosponge Pseudoceratina arabica (Pseudoceratinidae, Verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers (Basel) 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Zółtowska-Aksamitowska, S.; Shaala, L.A.; Youssef, D.T.A.; Elhady, S.S.; Tsurkan, M.V.; Petrenko, I.; Wysokowski, M.; Tabachnick, K.; Meissner, H.; Ivanenko, V.N.; et al. First report on chitin in a non-verongiid marine demosponge: The Mycale euplectellioides case. Mar. Drugs 2018, 16, 68. [Google Scholar] [CrossRef]
- Żółtowska-Aksamitowska, S.; Tsurkan, M.V.; Lim, S.C.; Meissner, H.; Tabachnick, K.; Shaala, L.A.; Youssef, D.T.A.; Ivanenko, V.N.; Petrenko, I.; Wysokowski, M.; et al. The demosponge Pseudoceratina purpurea as a new source of fibrous chitin. Int. J. Biol. Macromol. 2018, 112, 1021–1028. [Google Scholar] [CrossRef]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef]
- Stepniak, I.; Galinski, M.; Nowacki, K.; Wysokowski, M.; Jakubowska, P.; Bazhenov, V.V.; Leisegang, T.; Ehrlich, H.; Jesionowski, T. A novel chitosan/sponge chitin origin material as a membrane for supercapacitors-preparation and characterization. RSC Adv. 2016, 6, 4007–4013. [Google Scholar] [CrossRef]
- Ehrlich, H. Biomimetic potential of chitin-based composite biomaterials of poriferan origin. In Biomimetic Biomaterials: Structure and Applications; Ruys, A.J., Ed.; Woodhead Publishing Limited, E-Publishing Inc.: Cambridge, UK, 2013; pp. 46–66. [Google Scholar]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef]
- Wysokowski, M.; Materna, K.; Walter, J.; Petrenko, I.; Stelling, A.L.; Bazhenov, V.V.; Klapiszewski, Ł.; Szatkowski, T.; Lewandowska, O.; Stawski, D.; et al. Solvothermal synthesis of hydrophobic chitin-polyhedral oligomeric silsesquioxane (POSS) nanocomposites. Int. J. Biol. Macromol. 2015, 78, 224–229. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Wysokowski, M.; Szalaty, T.J.; Jesionowski, T.; Motylenko, M.; Rafaja, D.; Koltsov, I.; Stöcker, H.; Bazhenov, V.V.; Ehrlich, H.; Stelling, A.L.; et al. Extreme biomimetic approach for synthesis of nanocrystalline chitin-(Ti,Zr)O2 multiphase composites. Mater. Chem. Phys. 2017, 188, 115–124. [Google Scholar] [CrossRef]
- Steck, E.; Burkhardt, M.; Ehrlich, H.; Richter, W. Discrimination between cells of murine and human origin in xenotransplants by species specific genomic in situ hybridization. Xenotransplantation 2010, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin of Poriferan Origin as a Unique Biological Material. In Blue Biotechnology: Production and Use of Marine Molecules; La Barre, S., Bates, S.S., Eds.; Wiley-VCH: Weinheim, Germany, 2018; pp. 821–854. [Google Scholar]
- Ehrlich, H.; Maldonado, M.; Parker, A.R.; Kulchin, Y.N.; Schilling, J.; Köhler, B.; Skrzypczak, U.; Simon, P.; Reiswig, H.M.; Tsurkan, M.V.; et al. Supercontinuum generation in naturally occurring glass sponges spicules. Adv. Opt. Mater. 2016, 4, 1608–1613. [Google Scholar] [CrossRef]
- Vette, R.S.; Rust, M.K. Periodicity of molting and resumption of post-molt feeding in the brown recluse spider Loxosceles reclusa (Araneae: Sicariidae). J. Kansas Entomol. Soc. 2010, 83, 306–312. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guan, K.; Bornstein, S.R.; Czaczyk, K.; Pokrovsky, O.; et al. Spider Chitin. The biomimetic potential and applications of Caribena versicolor tubular chitin. Carbohydr. Polym. 2019, 226, 115301. [Google Scholar] [CrossRef]
- Bagaturov, M.F.; Jammu, A. Some notes on breeding Avicularia versicolor with comments on the hobby in Russia. J. Br. Tarantula Soc. 2005, 20, 78–88. [Google Scholar]
- Global Tarantula Market. Available online: https://www.globaltarantulamarket.com/ (accessed on 15 July 2019).
- German Arachnologic Society. Available online: https://www.dearge.de/english.php (accessed on 15 July 2019).
- British Tarantula Society. Available online: https://www.thebts.co.uk/ (accessed on 15 July 2019).
- Platnick, N.I. World Spider Catalog (2019). Available online: http://wsc.nmbe.ch (accessed on 15 June 2019).
- Collatz, K.G.; Mommsen, T. Physiological conditions and variations of body constituents during the moulting cycle of the spider Tegenaria atrica C. L. Koch (agelenidae). Comp. Biochem. Physiol. Part A 1975, 52, 465–476. [Google Scholar] [CrossRef]
- Minch, E.W. The molting sequence in Aphonopelma chalcodes (Araneae: Theraphosidae). J. Arachnol. 1977, 5, 133–137. [Google Scholar]
- Nyffeler, M.; Birkhofer, K. An estimated 400–800 million tons of prey are annually killed by the global spider community. Sci. Nat. 2017, 104, 30. [Google Scholar] [CrossRef] [PubMed]
- Cloudsley-Thompson, J.L. Epicuticle of arthropods. Nature 1950, 165, 692–693. [Google Scholar] [CrossRef] [PubMed]
- Rudall, K.M. The chitin/protein complexes of Insect cuticles. Adv. Insect Physiol. 1963, 1, 257–313. [Google Scholar]
- Sewell, M.T. Pore canals in a spider cuticle. Nature 1951, 167, 857–858. [Google Scholar] [CrossRef]
- Sewell, M.T. The histology and histochemistry of the cuticle of a spider, Tegenaria domestica. Ann. Entomol. Soc. Am. 1955, 48, 107–117. [Google Scholar] [CrossRef]
- Nentwig, W. Ecophysiology of Spiders; Springer-Verlag: Berlin, Germany, 1987; pp. 3–30. [Google Scholar]
- Davies, G.; Knight, D.P.; Vollrath, F. Chitin in the silk gland ducts of the spider Nephilia edulis and silkworm Bombyx mori. PLoS ONE 2013, 8, e7322. [Google Scholar] [CrossRef]
- Hsiung, B.-K.; Blackledge, T.A.; Shawkey, M.D. Spiders do have melanin after all. J. Exp. Biol. 2015, 218, 3632–3635. [Google Scholar] [CrossRef]
- Tarangini, K.; Mishra, S. Production of melanin by soil microbial isolate on fruit waste extract: Two step optimization of key parameters. Biotechnol. Rep. 2014, 4, 139–146. [Google Scholar] [CrossRef]
- Casadevall, A. Melanin triggers antifungal defences. Nature 2018, 555, 319–320. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł. The isolation, purification and analysis of the melanin pigment extracted from Armillaria mellea rhizomorphs. World Sci. News 2018, 100, 135–153. [Google Scholar]
- Roy, S.; Rhim, J. Preparation of carrageenan-based functional nanocomposite films incorporated with melanin nanoparticles. Colloids Surf. B Biointerfaces 2019, 176, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zeng, Z.; Liu, L.; Chen, J.; Zhou, H.; Huang, L.; Huang, J.; Xu, H.; Xu, Y.; Chen, Z.; et al. Polydopamine nanoparticles for treatment of acute inflammation-induced injury. Nanoscale 2018, 10, 6981–6991. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications — cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef] [PubMed]
- Mbonyiryivuze, A.; Mwakikunga, B.; Dhlamini, S.M.; Maaza, M. Fourier transform infrared spectroscopy for Sepia melanin. Phys. Mater. Chem. 2015, 3, 25–29. [Google Scholar]
- Politi, Y.; Priewasser, M.; Pippel, E.; Zaslansky, P.; Hartmann, J.; Siegel, S.; Li, C.; Barth, F.G.; Fratzl, P. A Spider’s Fang: How to design an injection needle using chitin-based composite material. Adv. Funct. Mater. 2012, 22, 2519–2528. [Google Scholar] [CrossRef]
- Politi, Y.; Pippel, E.; Licuco-Massouh, A.C.J.; Bertinetti, L.; Blumtritt, H.; Barth, F.G.; Fratzl, P. Nano-channels in the spider fang for the transport of Zn ions to cross-link His-rich proteins pre-deposited in the cuticle matrix. Arthropod Struct. Dev. 2017, 46, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, C.S.; Bertani, R. Taxonomic revision and cladistic analysis of Avicularia Lamarck, 1818 (Araneae, Theraphosidae, Aviculariinae) with description of three new aviculariine genera. Zookeys 2017, 659, 1–185. [Google Scholar] [CrossRef]
- Bertani, R.; Boston, T.; Evenou, Y.; Huadanucci, J.P.L. Urticating hairs of Avicularia versicolor (Walckenaer, 1837) (Araneae, Theraphosidae). Br. Arachnol. Soc. 2003, 12, 395–398. [Google Scholar]
- Browning, H.C. The integument and moult cycle of Tegenaria atrica (Araneae). Proc. R. Soc. B 1942, 131, 65–86. [Google Scholar]
- Nemenz, H. Über den Bau der Kutikula und dessen Einfluß auf die Wasserabgabe bei Spinnen. Osterr Akad Wiss Math-Nat KI Abt I 1955, 164, 65–76. [Google Scholar]
- Barth, F.G. Die Feinstruktur des Spinneninteguments, I. Die Cuticula des Laufbeins adulter hiiutungsferner Tiere (Cupiennius salei Keys). Zeitschrift für Zellforschung und Mikroskopische Anatomie 1969, 97, 137–159. [Google Scholar] [CrossRef]
- Barth, F.G. Die Feinstruktur des Spinneninteguments - II. Die raumliche Anordnung der Mikrofasern in der lamellierten Cuticula und ihre Beziehung zur Gestalt der Porenkanale (Cupiennius salei Keys., adult, hautungsfern, Tarsus). Zeitschrift für Zellforschung und Mikroskopische Anatomie 1970, 104, 87–107. [Google Scholar] [CrossRef]
- Barth, F.G. Microfiber reinforcement of an arthropod cuticle. Cell Tissue Res. 1973, 144, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Hadley, N.F. Cuticular permeability and lipid composition of the black widow spider, Latrodectus hesperus. Proc. R. Soc. B 1978, 42, 429–438. [Google Scholar]
- Hadley, N.F. Fine structure of the cuticle of the vlack window spider with reference to surface lipids. Tissue Cell 1981, 13, 805–817. [Google Scholar] [CrossRef]
- Krishnakumaran, A. The early post-moult cuticle in Buthus. Q. J. Microsc. Sci. 1960, 101, 433–438. [Google Scholar]
- Krishnakumaran, A. A comparative study of the Atachnid cuticle. II Chemical nature. Zeitschrift fur vergleiehende Physiol. 1961, 44, 478–486. [Google Scholar] [CrossRef]
- Krishnakumaran, A. A comparative study of the cuticle in Arachnida. I. Structure and staining properties. Zool. Jahrb. Anat. 1962, 80, 49–64. [Google Scholar]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express hethod for isolation of ready-to-use 3D chitin scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef]
- Kintsu, H.; Okumura, T.; Negishi, L.; Ifuku, S.; Kogure, T.; Sakuda, S.; Suzuki, M. Chitin degraded by chitinolytic enzymes induces crystal defects of calcites. In Biomineralization; Endo, K., Kogure, T., Nagasawa, H., Eds.; Springer: Tokyo, Japan, 2018; pp. 375–381. [Google Scholar]
- Yeng, Y.A.L.; Kadir, S.M.A.; Ghazali, H.M.; Rahman, R.N.Z.R.; Saari, N. A comparative study of extraction techniques for maximum recovery of glutamate decarboxylase (GAD) from Aspergillus oryzae NSK. BMC Res. Notes 2013, 6, 526. [Google Scholar]
- Ehrlich, H.; Wysokowski, M.; Zółtowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T. Collagens of poriferan origin. Mar. Drugs 2018, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Fromont, J.; Żółtowska-Aksamitowska, S.; Galli, R.; Meissner, H.; Erpenbeck, D.; Vacelet, J.; Diaz, C.; Tsurkan, M.V.; Petrenko, I.; Youssef, D.T.A.; et al. New family and genus of a Dendrilla-like sponge with characters of Verongiida. Part II. Discovery of chitin in the skeleton of Ernstilla lacunosa. Zool. Anz. 2019, 280, 21–29. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Shang, S.; Zhu, L.; Fan, J. Intermolecular interactions between natural polysaccharides and silk fibroin protein. Carbohydr. Polym. 2013, 93, 561–573. [Google Scholar] [CrossRef]
- Machovic, V.; Lapcak, L.; Havelcova, M.; Borecka, L.; Novotna, M.; Novotna, M.; Javurkova, I.; Langrova, I.; Hajkova, S.; Brozova, A.; et al. Analysis of European honeybee (Apis mellifera) wings using ATR-FTIR and Raman spectroscopy: A pilot study. Sci. Agric. Bohem. 2017, 48, 22–29. [Google Scholar] [CrossRef]
- Focher, B.; Naggi, A.; Torri, G.; Cossani, A.; Terbojevich, M. Structural differences between chitin polymorphs and their precipitates from solutions – evidence from CP-MAS 13C NMR, FT-IR and FT-Raman spectroscopy. Carbohydr. Polym. 1992, 17, 97–102. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Noishiki, Y.; Wada, M. X-ray structure of anhydrous β-chitin at 1A resolution. Macromolecules 2011, 44, 950–957. [Google Scholar] [CrossRef]
- Jang, M.; Kong, B.; Jeong, Y.; Lee, C.H.; Nah, J. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Sikorski, P.; Hori, R.; Wada, M. Revisit of α-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009, 10, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, H.G. Fine structure of viscose rayon. J. Appl. Phys. 1946, 17, 482. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, J.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1958, 29, 786–794. [Google Scholar] [CrossRef]
- Struszczyk, H. Microcrystalline chitosan. I. Preparation and properties of microcrystalline chitosan. J. Appl. Polym. Sci. 1987, 33, 177–189. [Google Scholar] [CrossRef]
- Focher, B.; Beltrame, P.L.; Naggi, A.; Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Hong, A.S.; Yang, Q.; Yuan, Y.; Chen, L.; Song, Y.; Lian, H. Sustainable co-solvent induced one step extraction of low molecular weight chitin with high purity from raw lobster shell. Carbohydr. Polym. 2019, 205, 236–243. [Google Scholar] [CrossRef]
- Andrade, C.T.; Silva, K.M.P.; Tavares, M.I.; Simao, R.A.; Achete, C.; Perez, C.A. Comparative study on structural features of α chitin from Xiphopenaeus kroyeri and its precipitated product from phosporic acid solution. J. Appl. Polym. Sci. 2001, 83, 151–159. [Google Scholar] [CrossRef]
- Neinhuis, C.; Nickerl, J.; Tsurkan, M.; Werner, C.; Werner, C. The multi-layered protective cuticle of Collembola: A chemical analysis. J. R. Soc. Interface 2014, 11, 20140619. [Google Scholar]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Petrášek, Z.; Pisera, A.; Simon, P.; Sivkov, V.N.; et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 17–20. [Google Scholar] [CrossRef]
- Domszya, J.G.; Roberts, G.A.F. Evaluation of infrared spectroscopic techniques for analysing chitosan. Macromol. Chem. Phys. 1985, 186, 1671–1677. [Google Scholar] [CrossRef]
- Knidri, H.E.L.; Khalfaouy, R.E.L.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process. Saf. Environ. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Małecki, P.H.; Raczynska, J.; Vorgias, C.E.; Rypniewski, W. Structure of a complete four-domain chitinase from Moritella marina, a marine psychrophilic bacterium. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Baharidis, P.K.; Georgoulis, A.; Engel, M.; Louka, M.; Karamolegkou, G.; Tsoka, A.; Blom, J.; Pot, B.; Malecki, P.; et al. Analysis of the complete genome sequence of the archaeon Pyrococcus chitonophagus DSM 10152 (formerly Thermococcus chitonophagus). Extremophiles 2016, 20, 351–361. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Schimpf, C.; Rafaja, D.; Brendler, E.; Viehweger, C.; Żółtowska-Aksamitowska, S.; Petrenko, I.; et al. Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena versicolor Spider Molt Cuticle. Molecules 2019, 24, 3736. https://doi.org/10.3390/molecules24203736
Machałowski T, Wysokowski M, Tsurkan MV, Galli R, Schimpf C, Rafaja D, Brendler E, Viehweger C, Żółtowska-Aksamitowska S, Petrenko I, et al. Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena versicolor Spider Molt Cuticle. Molecules. 2019; 24(20):3736. https://doi.org/10.3390/molecules24203736
Chicago/Turabian StyleMachałowski, Tomasz, Marcin Wysokowski, Mikhail V. Tsurkan, Roberta Galli, Christian Schimpf, David Rafaja, Erica Brendler, Christine Viehweger, Sonia Żółtowska-Aksamitowska, Iaroslav Petrenko, and et al. 2019. "Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena versicolor Spider Molt Cuticle" Molecules 24, no. 20: 3736. https://doi.org/10.3390/molecules24203736








