Atomic Force Microscope Guided SERS Spectra Observation for Au@Ag-4MBA@PVP Plasmonic Nanoparticles
Abstract
:1. Introduction
2. Results
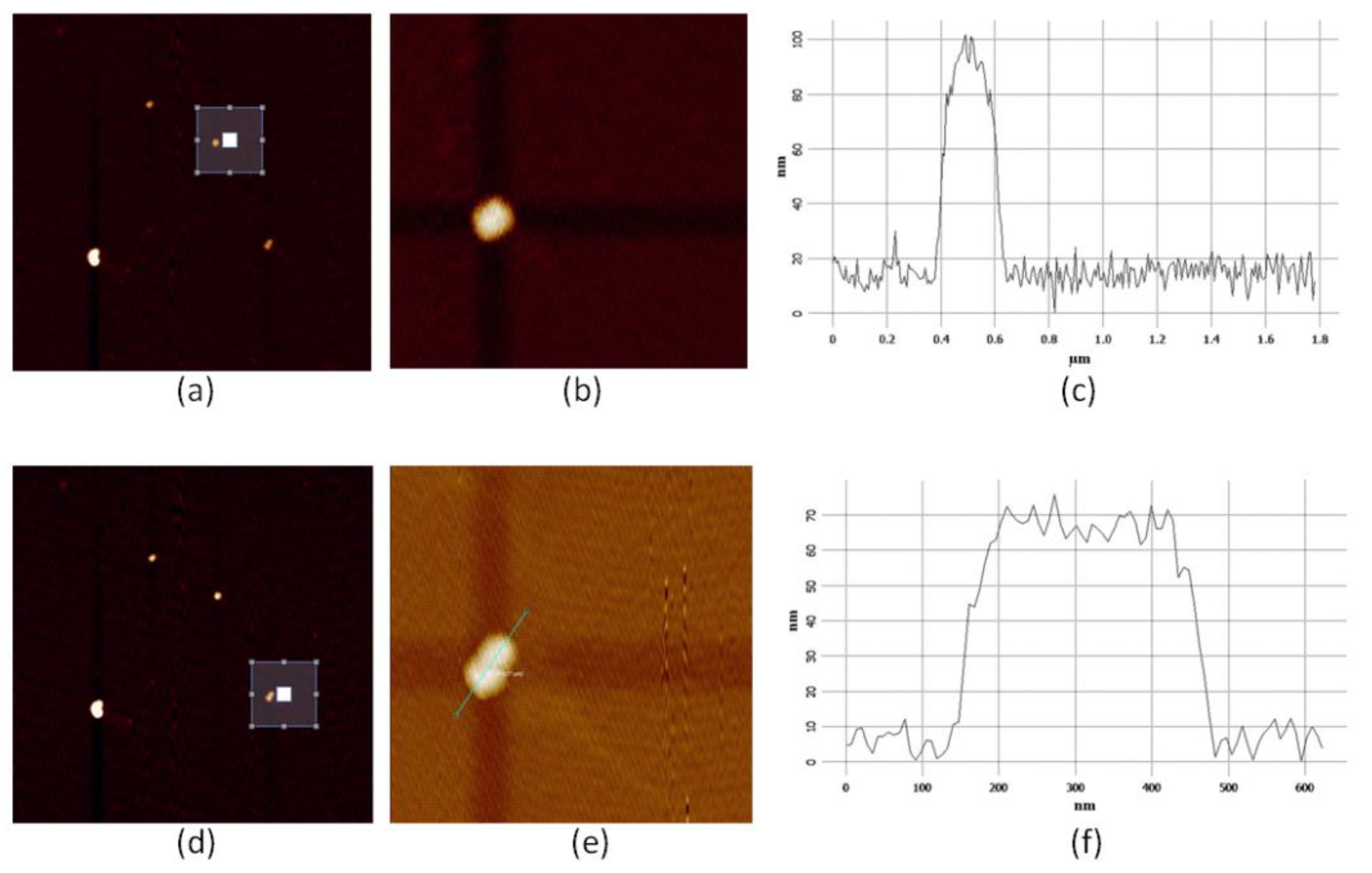
2.1. Atom Force Microscope Images and SERS Spectra
2.2. Observation of Abnormal SERS Spectra for Dimer Structures
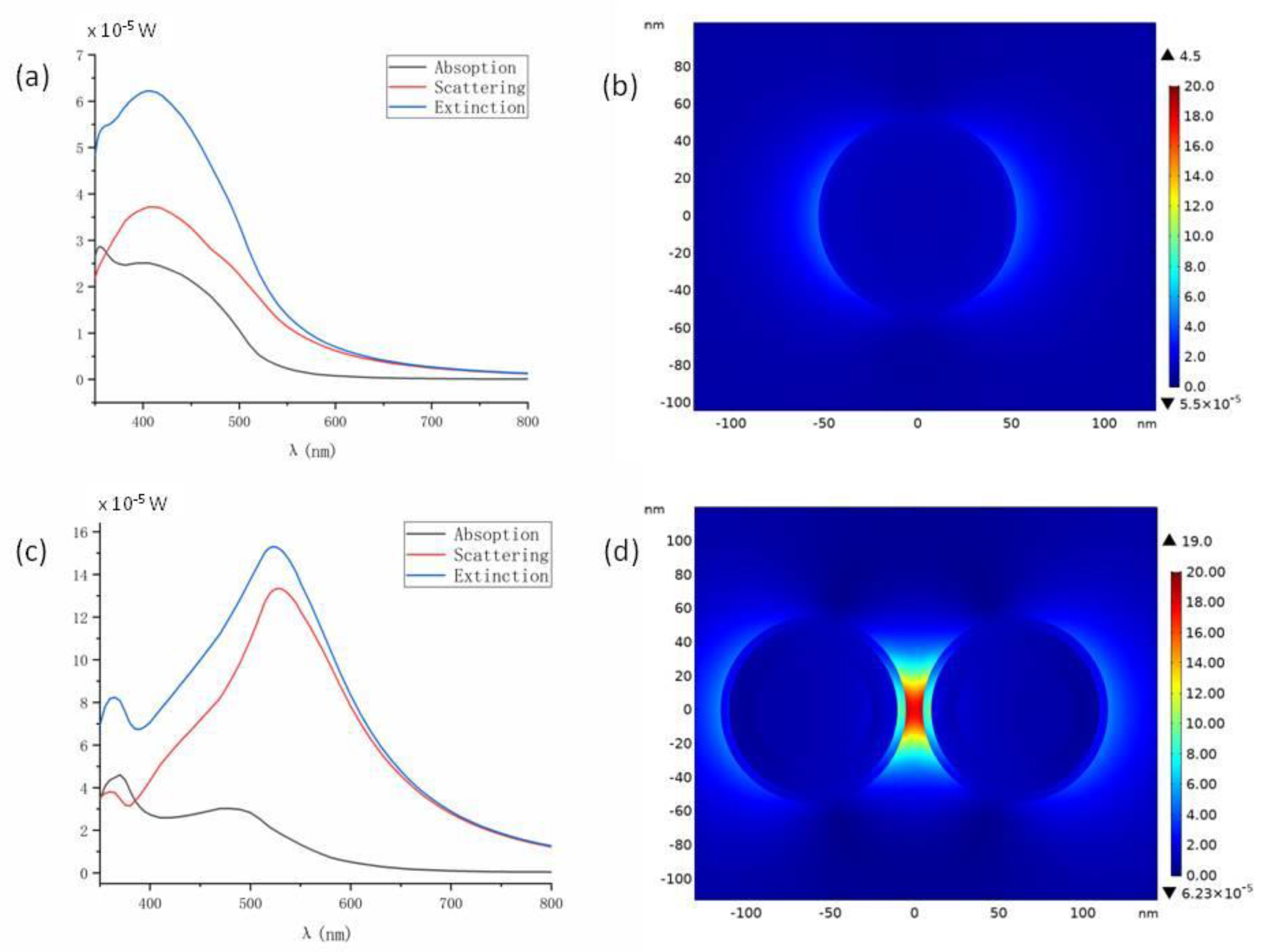
2.3. FEM Simulation of SERS Enhancement Factors and Photothermal Temperature Increase
3. Discussion
4. Materials and Methods
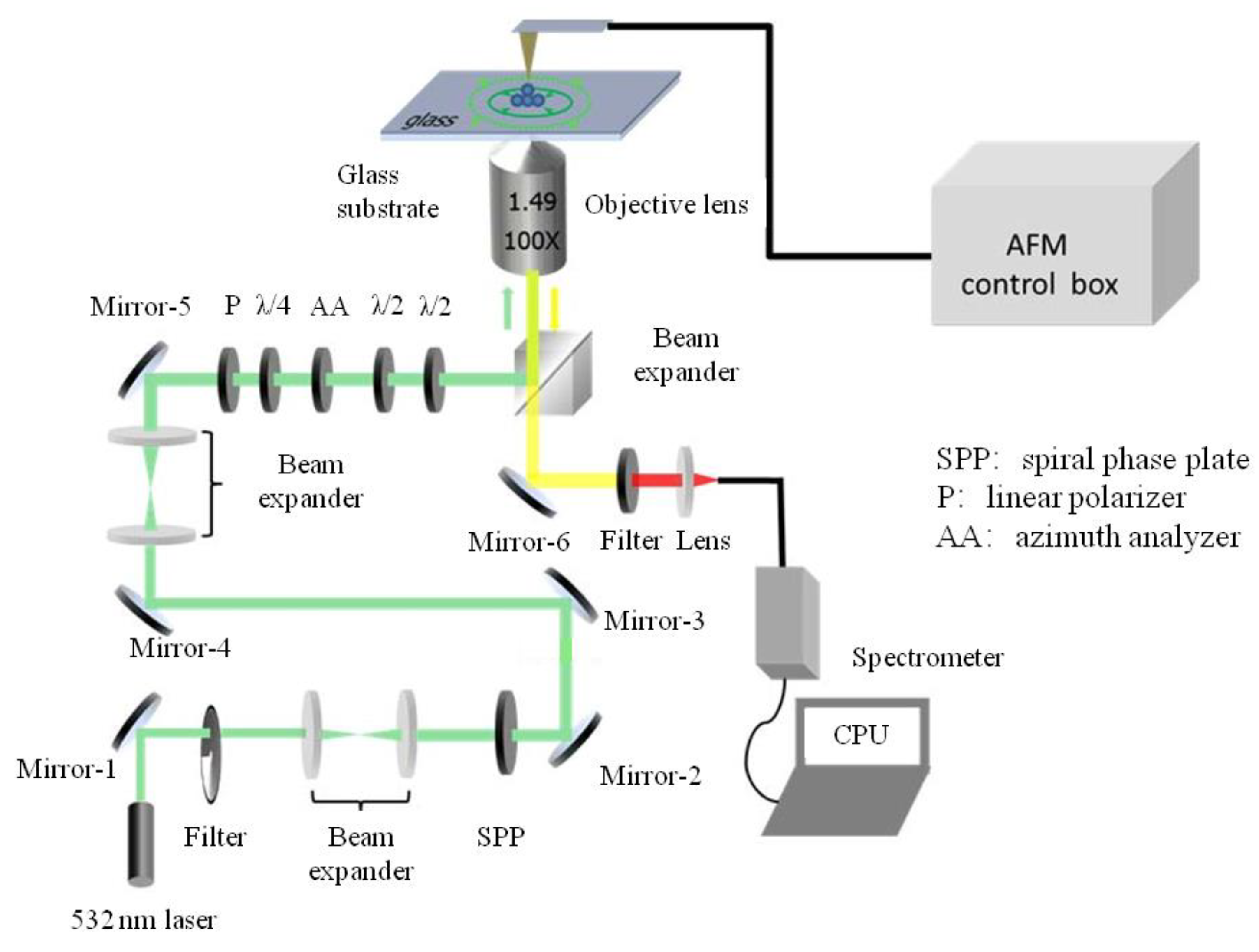
4.1. Experimental System
4.2. Sample Preparation
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.F.; Zhang, Y.J.; Panneerselvam, R.; Tian, Z.Q. Core-shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 2017, 117, 5002–5019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zong, S.F.; Zhu, D.; Cui, Y.P. SERS-activated platforms for immunoassay: Probes, encoding, methods, and applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.X.; Chen, T.; Lau, W.S.; Wang, Y.; Tang, Q.H.; Yang, Y.H.; Chen, H.Y. Development of polymer-encapsulated metal nanoparticles as surface-enhanced Raman scattering probes. Small 2009, 2, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.B.; Wang, Z.Y.; Yang, J.; Song, C.Y.; Zhang, R.H.; Cui, Y.P. Polyvinylpyrrolidone- (PVP-) coated silver aggregates for high performance surface-enhanced Raman scattering in living cells. Nanotechnology 2009, 20, 445102. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, K.S.; Polavarapu, L.; Sergio, R.C.; Luis, M.L.M.; Jorge, P.J.; Isabel, P.S. Size tunable Au@Ag core-shell nanoparticles: Synthesis and surface-enhanced Raman scattering properties. Langmuir 2013, 29, 15076–15082. [Google Scholar]
- Jaroslow, G.; Frank, W.D.; Namboodiri, P.; Doo, I.K.; Vincent, A.H.; Robert, F.C. Size measurement of nanoparticles using atomic force microscopy. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Clifton, NJ, USA, 2011; pp. 71–82. [Google Scholar]
- Nie, S.M.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Bjerneld, E.J.; Kall, M.; Borjesson, L. Spectroscopy of single hemoglobin molecules by surface enhanced Raman scattering. Phys. Rev. Lett. 1999, 83, 4357–4360. [Google Scholar] [CrossRef]
- Lim, D.L.; Jeon, K.S.; Kim, H.M.; Nam, J.M.; Suh, Y.D. Nanogap-engineerable Raman-active nanodumbbells for single-molecule detection. Nat. Mat. 2010, 9, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Pashaee, F.; Hou, R.J.; Gobbo, P.; Workentin, M.S.; Francois, L.L. Tip-enhanced Raman spectroscopy of self-assembled thiolated monolayers on flat gold nanoplates using Gaussian-transverse and radially polarized excitations. J. Phys. Chem. C 2013, 117, 15639–15646. [Google Scholar] [CrossRef]
- Kim, J.H.; Twaddle, K.M.; Cermark, L.M.; Jiang, W.; Yun, J.H.; Byun, H. Photothermal heating property of gold nanoparticle loaded substrates and their SERS response. Colloids Surf. A Physicochem. Eng. Asp. 2016, 498, 20–29. [Google Scholar] [CrossRef]
- Yang, L.B.; Jiang, X.; Ruan, W.D.; Zhao, B.; Xu, W.Q.; Lombardi, J.R. Adsorption study of 4-MBA on TiO2 nanoparticles by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 2004–2008. [Google Scholar] [CrossRef]
- Gehan, H.; Fillaud, L.; Chehimi, M.M.; Aubard, J.; Hohenau, A.; Felidj, N.; Mangeney, C. Thermo-induced electromagnetic coupling in gold/polymer hybrid plasmonic structures probed by surface-enhanced Raman scattering. ACS Nano 2010, 11, 6491–6500. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Cole, R.M.; Speed, J.D.; Pelfrey, S.H.; Russell, A.E.; Bartlett, P.N.; Barnett, S.M.; Baumberg, J.J. Understanding the surface-enhanced Raman spectroscopy “background”. J. Phys. Chem. C 2010, 114, 7242–7250. [Google Scholar] [CrossRef]
- Hugall, J.T.; Baumberg, J.J. Demonstrating photoluminescence from Au is electronic inelastic light scattering of a plasmonic metal: The origin of SERS backgrounds. Nano Lett. 2015, 15, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Q.; Yi, J.; Zhong, J.H.; Hu, S.; Liu, B.J.; Liu, J.Y.; Zong, C.; Lei, Z.C.; Wang, X.; Aizpurua, J.; et al. Plasmonic photoluminescence for recoving native chemical information from surface-enhanced Raman scattering. Nat. Commun. 2017, 8, 14891. [Google Scholar] [CrossRef] [PubMed]
- Chala, T.F.; Wu, C.M.; Chou, M.H.; Gebeyehu, M.B.; Cheng, K.B. High efficient near infrared photothermal conversion properties of reduced tungsten oxide/polyurethane nanocomposites. Nanomaterials 2017, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Bassi, B.; Dacarro, G.; Galinetto, P.; Marchesi, N.; Pallavicini, P.; Pascale, A.; Pervesi, S.; Taglietti, A. Tailored coating of gold nanostars: Rational approach to prototype of theranostic device based on SERS and photothermal effects at ultralow irradiance. Nanotechnology 2018, 8, 235301. [Google Scholar] [CrossRef] [PubMed]
- Borodko, Y.; Habas, S.E.; Koebel, M.; Yang, P.D.; Frei, H.; Somorjai, G.A. Probing the interaction of poly(vinylpyrrolidone) with platinum nanocrystals by UV-Raman and FTIR. J. Phys. Chem. B 2006, 110, 23052–23059. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Xu, L.; Wu, X.; Fang, H.; Zhong, S.; Wang, Z.; Bu, J.; Yuan, X. Atomic Force Microscope Guided SERS Spectra Observation for Au@Ag-4MBA@PVP Plasmonic Nanoparticles. Molecules 2019, 24, 3789. https://doi.org/10.3390/molecules24203789
Yang L, Xu L, Wu X, Fang H, Zhong S, Wang Z, Bu J, Yuan X. Atomic Force Microscope Guided SERS Spectra Observation for Au@Ag-4MBA@PVP Plasmonic Nanoparticles. Molecules. 2019; 24(20):3789. https://doi.org/10.3390/molecules24203789
Chicago/Turabian StyleYang, Liu, Libei Xu, Xiuju Wu, Hui Fang, Shenfei Zhong, Zhuyuan Wang, Jing Bu, and Xiaocong Yuan. 2019. "Atomic Force Microscope Guided SERS Spectra Observation for Au@Ag-4MBA@PVP Plasmonic Nanoparticles" Molecules 24, no. 20: 3789. https://doi.org/10.3390/molecules24203789





