Experimental Design Modeling of the Effect of Hexagonal Wurtzite—ZnO Synthesis Conditions on Its Characteristics and Performance as a Cationic and Anionic Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zinc Hydroxide
2.3. Hydrothermal Treatment
2.4. Washing and Drying
2.5. Methyl Orange and Methylene Blue Solution Preparation
2.6. Characterization
2.7. Point of Zero Charge
2.8. Adsorption Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duo, S.; Li, Y.; Liu, Z.; Zhong, R.; Liu, T. Novel hybrid self-assembly of an ultralarge ZnO macroflower and defect intensity-induced photocurrent and photocatalytic properties by facile hydrothermal synthesis using CO(NH2)2–N2H4 as alkali sources. Mater. Sci. Semicond. Process. 2016, 56, 196–212. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.R.; Nascimento, C.C.; Lima, Z.M.; Costa, L.P.; Gimenez, I.F.; Teixeira-Neto, E. Star-shaped ZnO/Ag hybrid nanostructures for enhanced photocatalysis and antibacterial activity. Appl. Surf. Sci. 2017, 399, 573–582. [Google Scholar] [CrossRef]
- Fiedot, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Lewińska, A.; Teterycz, H. The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes. Materials 2017, 10, 353. [Google Scholar] [CrossRef]
- Ali, T.T.; Narasimharao, K.; Parkin, I.P.; Carmalt, C.J.; Sathasivam, S.; Basahel, S.N.; Bawaked, S.M.; Al-Thabaiti, S.A. Effect of pretreatment temperature on the photocatalytic activity of microwave irradiated porous nanocrystalline ZnO. New J. Chem. 2015, 39, 321–332. [Google Scholar] [CrossRef]
- Anandan, M.; Dinesh, S.; Krishnakumar, N.; Balamurugan, K. Tuning the crystalline size of template free hexagonal ZnO nanoparticles via precipitation synthesis towards enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2017, 28, 2574–2585. [Google Scholar] [CrossRef]
- Tabib, A.; Bouslama, W.; Sieber, B.; Addad, A.; Elhouichet, H.; Férid, M.; Boukherroub, R. Structural and optical properties of Na doped ZnO nanocrystals: Application to solar photocatalysis. Appl. Surf. Sci. 2017, 396, 1528–1538. [Google Scholar] [CrossRef]
- Jo, W.; Tayade, R.J. New Generation Energy-E ffi cient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic Decolorization of Dye with Self-Dye-Sensitization under Fluorescent Light Irradiation. ChemEngineering 2017, 1, 8. [Google Scholar] [CrossRef]
- Bhakat, C.; Singh, P.P. Zinc Oxide Nanorods: Synthesis and Its Applications in Solar Cell. Int. J. Mod. Eng. Res. 2012, 2, 2452–2454. [Google Scholar]
- Oprea, O.; Andronescu, E.; Ficai, D.; Ficai, A.; Oktar, F.; Yetmez, M. ZnO Applications and Challenges. Curr. Org. Chem. 2014, 18, 192–203. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, H.; Zhang, W.; Zhou, T.; Qiu, Y.; Xu, K.; Zhang, B.; Yang, H. Synthesis and characterization of twinned flower–like ZnO structures grown by hydrothermal methods. Ceram. Int. 2016, 42, 9648–9652. [Google Scholar] [CrossRef]
- Joo, J.; Chow, B.Y.; Prakash, M.; Boyden, E.S.; Jacobson, J.M. Face-selective electrostatic control of hydrothermal zinc oxide nanowire synthesis. Nat. Mater. 2011, 10, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanlary, M.R.; Vahedi, V.; Reyhani, A. Synthesis and Characterization of ZnO Nanowires by Thermal Oxidation of Zn Thin Films at Various Temperatures. Molecules 2012, 17, 5021–5029. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Wicker, S.; Wang, X.; Erichsen, E.; Fu, F. Organozinc Precursor-Derived Crystalline ZnO Nanoparticles: Synthesis, Characterization and Their Spectroscopic Properties. Nanomaterials 2018, 8, 22. [Google Scholar] [CrossRef]
- Moezzi, A.; Cortie, M.; McDonagh, A. Aqueous pathways for the formation of zinc oxide nanoparticles. Dalt. Trans. 2011, 40, 4871. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Aziz, S.H.A.; Zakaria, A.; Ghazali, M.S.M. Effect of ZnO on the Physical Properties and Optical Band Gap of Soda Lime Silicate Glass. Int. J. Mol. Sci. 2012, 13, 7550–7558. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, M.; Saroja, M.; Kumar, V.R.; Jenifer, G. Particle Size Analysis for Different Substrate of ZnS/ZnO Thin Film in CBD Technique. Int. J. Thin Films Sci. Technol. 2016, 5, 137–141. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Majid, W.A.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [Green Version]
- Balogun, S.W.; Sanusi, Y.K.; Aina, A.O. Impact of Post-Deposition Heat Treatment on the Morphology and Optical Properties of Zinc Oxide (ZnO) Thin Film Prepared by Spin-Coating Technique. J. Photon. Mater. Technol. 2017, 3, 20. [Google Scholar] [CrossRef]
- Matei, A.; Tucureanu, V.; Dumitrescu, L. Aspects Regarding Synthesis And Applications Of ZnO. Bull. Transilv. Univ. Brasov. Eng. Sci. 2014, 7, 45–52. [Google Scholar]
- Tombácz, E. Ph-dependent surface charging of metal oxides. Period. Polytech. Chem. Eng. 2009, 53, 77–86. [Google Scholar] [CrossRef]
- Sathya, M.; Claude, A.; Govindasamy, P.; Sudha, K.; Claude, A. Growth of pure and doped ZnO thin films for solar cell applications. Pelagia Res. Libr. Adv. Appl. Sci. Res. 2012, 3, 2591–2598. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons: Phoenix, AZ, USA, 2017. [Google Scholar]
- Prouzet, E.; Hessien, M.; Singh, N.; Kim, C. Stability and tunability of O/W nanoemulsions prepared by phase inversion composition. Langmuir 2011, 27, 2299–2307. [Google Scholar]
- Hossini, H.; Safari, M.; Rezaee, R.; Darvishi, R.; Soltani, C. Application of experimental design approach for optimization of the photocatalytic degradation of humic substances in aqueous solution using immobilized ZnO nanoparticles. J. Adv. Environ. Health Res. 2015, 3, 154–163. [Google Scholar]
- Ibupoto, Z.H.; Khun, K.; Eriksson, M.; AlSalhi, M.; Atif, M.; Ansari, A.; Willander, M. Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles. Materials 2013, 6, 3584–3597. [Google Scholar] [CrossRef]
- Lu, C.; Yeh, C. Influence of hydrothermal conditions on the morphology and particle size of zinc oxide powder. Ceram. Int. 2000, 26, 351–357. [Google Scholar] [CrossRef]
- Kumar, D.; Dubey, K.K. Optimization of Zinc Oxide nanoparticles synthesis to fabricate glucose oxidase sensor. Pelagia Res. Libr. 2012, 3, 3081–3088. [Google Scholar]
- Lee, K.M.; Hamid, S.B.A. Simple Response Surface Methodology: Investigation on Advance Photocatalytic Oxidation of 4-Chlorophenoxyacetic Acid Using UV-Active ZnO Photocatalyst. Materials 2015, 8, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Adiga, N.; Ba, S.; Dasgupta, T.; Wu, C.F.J.; Wang, Z.L. Optimizing and Improving the Growth Quality of ZnO Nanowire Arrays Guided by Statistical Design of Experiments. ACS Nano 2009, 3, 1803–1812. [Google Scholar] [CrossRef]
- Jain, R.; Sikarwar, S. Photocatalytic and adsorption studies on the removal of dye Congo red from wastewater. Int. J. Environ. Pollut. 2006, 27, 158. [Google Scholar] [CrossRef] [Green Version]
- Darvishi, R.; Cheshmeh, S.; Rezaee, A.; Rezaee, R.; Safari, M. Photocatalytic degradation of methylene blue dye over immobilized ZnO nanoparticles: Optimization of calcination conditions. J. Adv. Environ. Health Res. 2015, 3, 1–7. [Google Scholar]
- Ali, A.H.; Naser, G.F.; Mohammed, S.A. Photocatalytic Degradation of Methyl Orange Dye using Different Photocatalysts under Solar Light. Int. J. ChemTech. Res. 2016, 9, 157–165. [Google Scholar]
- Chebor, L.J. Characterization of Synthesized ZnO Nanoparticles and their Application in Photodegradation of Methyl Orange Dye Under Fluorescent Lamp Irradiation. Int. J. Sci. Eng. Sci. 2018, 2, 5–8. [Google Scholar]
- Sani, H.A.; Aliyu, H.S.; Tukur, S.A. Methylene Blue Dye Adsorption Using a Polymer Coated ZnO with Chitosan Nano-Catalyst. J. Appl. Chem. 2015, 8, 34–38. [Google Scholar]
- Saleh, K.A.; Al-taie, H.H. Methylene Blue Adsorption Study Using Different Zno Types ( Normal, Shaheed Factory, Nano ). Int. J. Sci. Res. 2017, 6, 939–944. [Google Scholar]
- Kulkarni, J.C.A.V.; Chavhan, A.; Bappakhane, A. Chimmankar ZnO Nanoparticles as Adsorbent for Removal of Methylene Blue dye. Res. J. Chem. Environ. Sci. 2016, 4, 158–163. [Google Scholar]
- Zhang, F.; Lan, J.; Yang, Y.; Wei, T.; Tan, R.; Song, W. Adsorption behavior and mechanism of methyl blue on zinc oxide nanoparticles. J. Nanoparticle Res. 2013, 15, 2034. [Google Scholar] [CrossRef]
- Al-Khateeb, I.; Mahmood, M. Adsorption, Thermodynamics and Kinetics of Methylene Blue on Nano Structured ZnO Crystals. Am. Chem. Sci. J. 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Tajizadegan, H.; Jafari, M.; Rashidzadeh, M.; Saffar-Teluri, A. A high activity adsorbent of ZnO–Al2O3 nanocomposite particles: Synthesis, characterization and dye removal efficiency. Appl. Surf. Sci. 2013, 276, 317–322. [Google Scholar] [CrossRef]
- Hessien, M.; Da’Na, E.; Al-Amer, K.; Khalaf, M.M.; D’Na, E. Nano ZnO (hexagonal wurtzite) of different shapes under various conditions: Fabrication and characterization. Mater. Res. Express 2019, 6, 085057. [Google Scholar] [CrossRef]
- Da’Na, E.; Sayari, A. Adsorption of copper on amine-functionalized SBA-15 prepared by co-condensation: Equilibrium properties. Chem. Eng. J. 2011, 166, 445–453. [Google Scholar] [CrossRef]
- Moharram, A.H.; Mansour, S.A.; Hussein, M.A.; Rashad, M. Direct Precipitation and Characterization of ZnO Nanoparticles. J. Nanomater. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Bodke, M.R.; Purushotham, Y.; Dole, B.N. Comparative study on zinc oxide nanocrystals synthesized by two precipitation methods. Cerâmica 2018, 64, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M.; Sotomayor, F. Characterization of Micro / Mesoporous Materials by Physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Ravikovitch, P.I.; Neimark, A.V. Diffusion-Controlled Hysteresis. Adsorption 2005, 11, 265–270. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Ma, X.; Ji, Y.; Xu, J.; Que, D. Synthesis of flower-like ZnO nanostructures by an organic-free hydrothermal process. Nanotechnology 2004, 15, 622–626. [Google Scholar] [CrossRef]
- Jiang, L.; Li, G.; Ji, Q.; Peng, H. Morphological control of flower-like ZnO nanostructures. Mater. Lett. 2007, 61, 1964–1967. [Google Scholar] [CrossRef]
- Shi, R.; Yang, P.; Dong, X.; Ma, Q.; Zhang, A. Growth of flower-like ZnO on ZnO nanorod arrays created on zinc substrate through low-temperature hydrothermal synthesis. Appl. Surf. Sci. 2013, 264, 162–170. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Stankovic, J.; Milonjic, S.; Zec, S. The influence of chemical and thermal treatment on the point of zero charge of hydrous zirconium oxide. J. Serbian Chem. Soc. 2013, 78, 987–995. [Google Scholar] [CrossRef]
- Ocholi, O.J.; Gimba, C.E.; Ndukwe, G.I.; Turoti, M.; Abechi, S.E.; Edogbanya, P.R.O. Effect of Time on the Adsorption of Methylene Blue, Methyl Orange and Indigo Carmine onto Activated Carbon. J. Appl. Chem. 2016, 9, 55–62. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



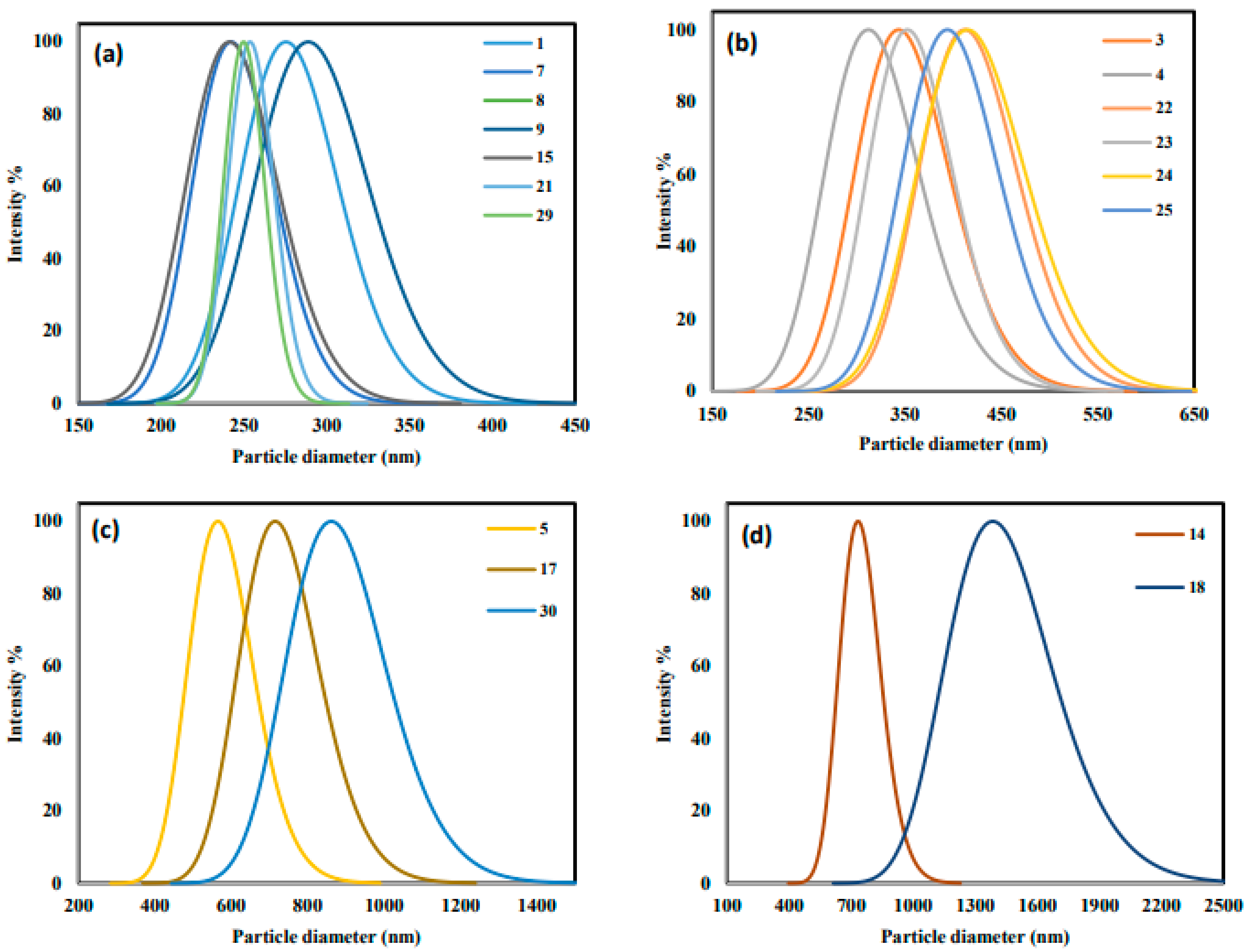


| Variable | Symbol | Level in Coded and Real Values | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Precursor concentration (M) | C | 0.2 | 0.3 | 0.4 |
| Temperature (°C) | T | 100 | 150 | 200 |
| Time (h) | t | 1 | 1.5 | 2 |
| pH | pH | 7 | 9 | 11 |
| Standard | Random | C (M) | pH | T (°C) | Time (h) | Point of Zero Charge (pHZC) | Dh (nm) | Adsorption Capacity of MB (qMB; mg/g) | Adsorption Capacity of MO (qMO; mg/g) | Removal Efficiency of MO (RMO; %) | Removal Efficiency of MB (RMB; %) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 0.2 | 7 | 100 | 1 | 7.3 | 279 | 5.15 | 5.78 | 72.30 | 64.41 |
| 2 | 6 | 0.4 | 7 | 100 | 1 | 5.9 | 1322 | 6.06 | 7.28 | 91.00 | 75.72 |
| 3 | 8 | 0.2 | 11 | 100 | 1 | 6.8 | 350 | 7.22 | 4.74 | 59.20 | 90.26 |
| 4 | 23 | 0.4 | 11 | 100 | 1 | 6.8 | 318 | 6.94 | 3.86 | 48.20 | 86.77 |
| 5 | 30 | 0.2 | 7 | 200 | 1 | 6.7 | 537 | 6.38 | 5.46 | 68.20 | 79.76 |
| 6 | 5 | 0.4 | 7 | 200 | 1 | 6 | 642 | 7.05 | 4.63 | 57.86 | 88.15 |
| 7 | 20 | 0.2 | 11 | 200 | 1 | 6.9 | 242 | 2.87 | 0.35 | 4.37 | 35.92 |
| 8 | 26 | 0.4 | 11 | 200 | 1 | 6.8 | 311 | 2.23 | 0.17 | 2.18 | 27.83 |
| 9 | 28 | 0.2 | 7 | 100 | 2 | 6.5 | 280 | 8.00 | 5.96 | 74.56 | 100.00 |
| 10 | 7 | 0.4 | 7 | 100 | 2 | 7.2 | 412 | 6.57 | 7.66 | 95.75 | 82.09 |
| 11 | 12 | 0.2 | 11 | 100 | 2 | 7.2 | 218 | 2.83 | 0.60 | 7.52 | 35.38 |
| 12 | 1 | 0.4 | 11 | 100 | 2 | 7.9 | 536 | 4.45 | 2.24 | 28.05 | 55.60 |
| 13 | 10 | 0.2 | 7 | 200 | 2 | 7.2 | 824 | 0.68 | 0.42 | 5.20 | 8.51 |
| 14 | 14 | 0.4 | 7 | 200 | 2 | 6.8 | 752 | 4.39 | 2.13 | 26.59 | 54.83 |
| 15 | 2 | 0.2 | 11 | 200 | 2 | 6.8 | 247 | 0.93 | 0.37 | 4.60 | 11.58 |
| 16 | 4 | 0.4 | 11 | 200 | 2 | 7.2 | 366 | 3.94 | 1.12 | 14.01 | 49.29 |
| 17 | 33 | 0.1 | 9 | 150 | 1.5 | 7.3 | 647 | 7.82 | 5.57 | 69.59 | 97.74 |
| 18 | 24 | 0.5 | 9 | 150 | 1.5 | 7.2 | 1252 | 7.17 | 6.39 | 79.87 | 89.65 |
| 19 | 29 | 0.3 | 5 | 150 | 1.5 | No precipitation was observed | |||||
| 20 | 25 | 0.3 | 13 | 150 | 1.5 | 8 | 8920 | 0.00 | 0.91 | 11.39 | 0.00 |
| 21 | 11 | 0.3 | 9 | 50 | 1.5 | 6.7 | 260 | 7.21 | 5.11 | 63.83 | 90.10 |
| 22 | 9 | 0.3 | 9 | 250 | 1.5 | 8.1 | 435 | 1.49 | 0.62 | 7.80 | 18.61 |
| 23 | 34 | 0.3 | 9 | 150 | 0.5 | 6.8 | 352 | 6.96 | 5.26 | 65.72 | 86.94 |
| 24 | 13 | 0.3 | 9 | 150 | 2.5 | 7.6 | 262 | 6.93 | 3.52 | 44.03 | 86.69 |
| 25 | 18 | 0.3 | 9 | 150 | 1.5 | 6.6 | 397 | 6.96 | 4.31 | 53.86 | 86.98 |
| 26 | 22 | 0.3 | 9 | 150 | 1.5 | 6.6 | 462 | 6.68 | 4.71 | 58.92 | 83.45 |
| 27 | 32 | 0.3 | 9 | 150 | 1.5 | 7.2 | 1475 | 6.48 | 5.36 | 67.06 | 80.98 |
| 28 | 21 | 0.3 | 9 | 150 | 1.5 | 6.6 | 417 | 7.24 | 4.83 | 60.43 | 90.54 |
| 29 | 15 | 0.3 | 9 | 150 | 1.5 | 7.1 | 437 | 7.86 | 4.51 | 56.32 | 98.30 |
| 30 | 3 | 0.3 | 7 | 150 | 1.5 | 7.2 | 851 | 8.00 | 6.24 | 77.95 | 100.00 |
| 31 | 19 | 0.3 | 11 | 150 | 1.5 | 8.1 | 373 | 4.70 | 1.71 | 21.41 | 58.74 |
| 32 | 16 | 0.4 | 9 | 150 | 1.5 | 6.9 | 472 | 7.86 | 5.16 | 64.48 | 98.19 |
| 33 | 17 | 0.2 | 9 | 150 | 1.5 | 7 | 497 | 6.44 | 5.03 | 62.93 | 80.49 |
| 34 | 31 | 0.3 | 9 | 250 | 2.5 | 6.5 | 937 | 0.24 | 0.62 | 7.76 | 3.05 |
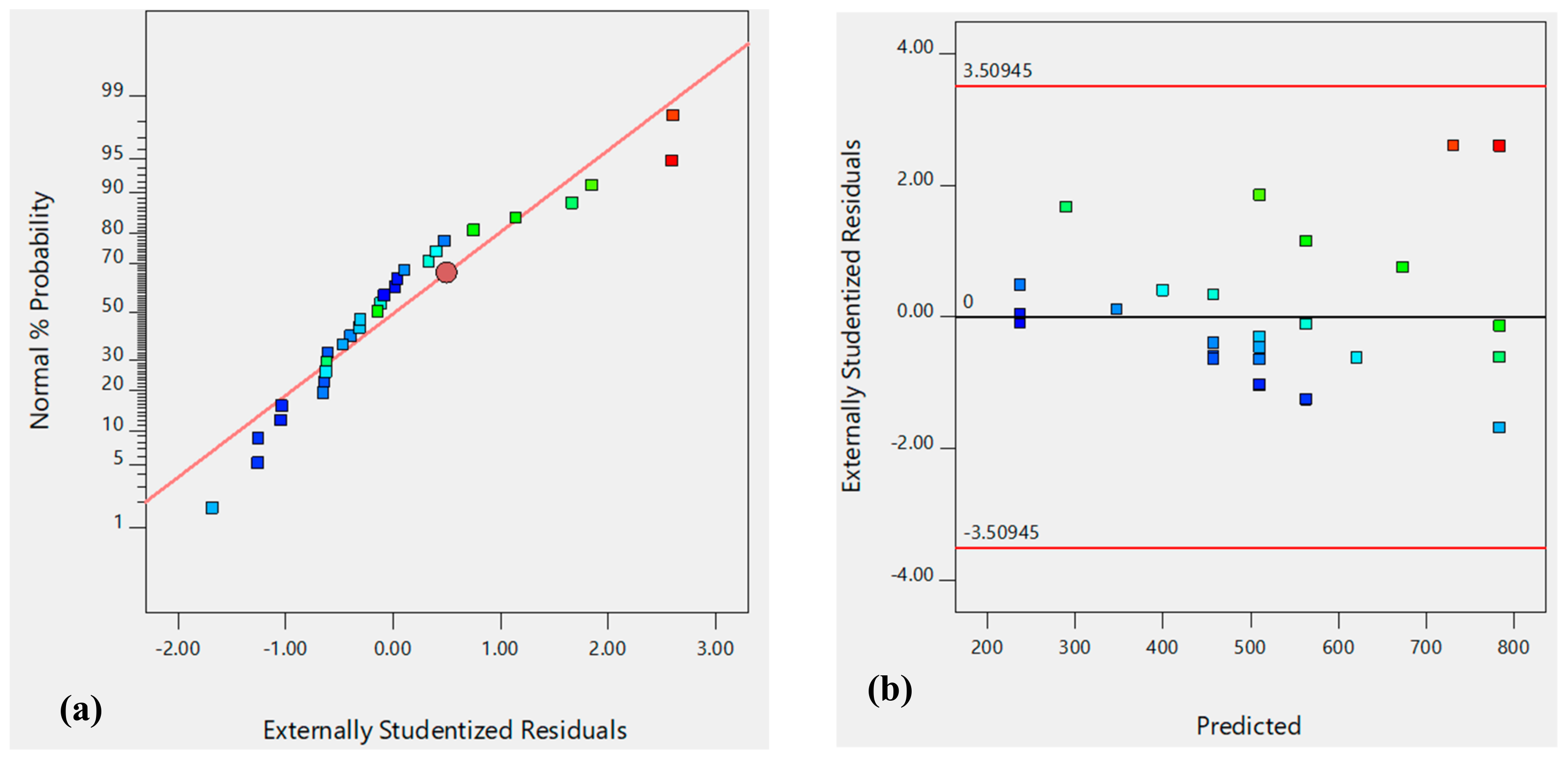
| Dh | R-sq | R-sq (adj) | R-sq (pred) | F-value | P-value | Residual F | Residual P |
| 0.3369 | 0.2858 | 0.1332 | 6.60 | 0.0048 | 78.23 | 0.0891 | |
| Uncoded equation: | Dh = +913.86185 + 1102.69231C − 81.56481pH | ||||||
| Coded equation: | Dh = +510.59 + 110.27C − 163.13pH | ||||||
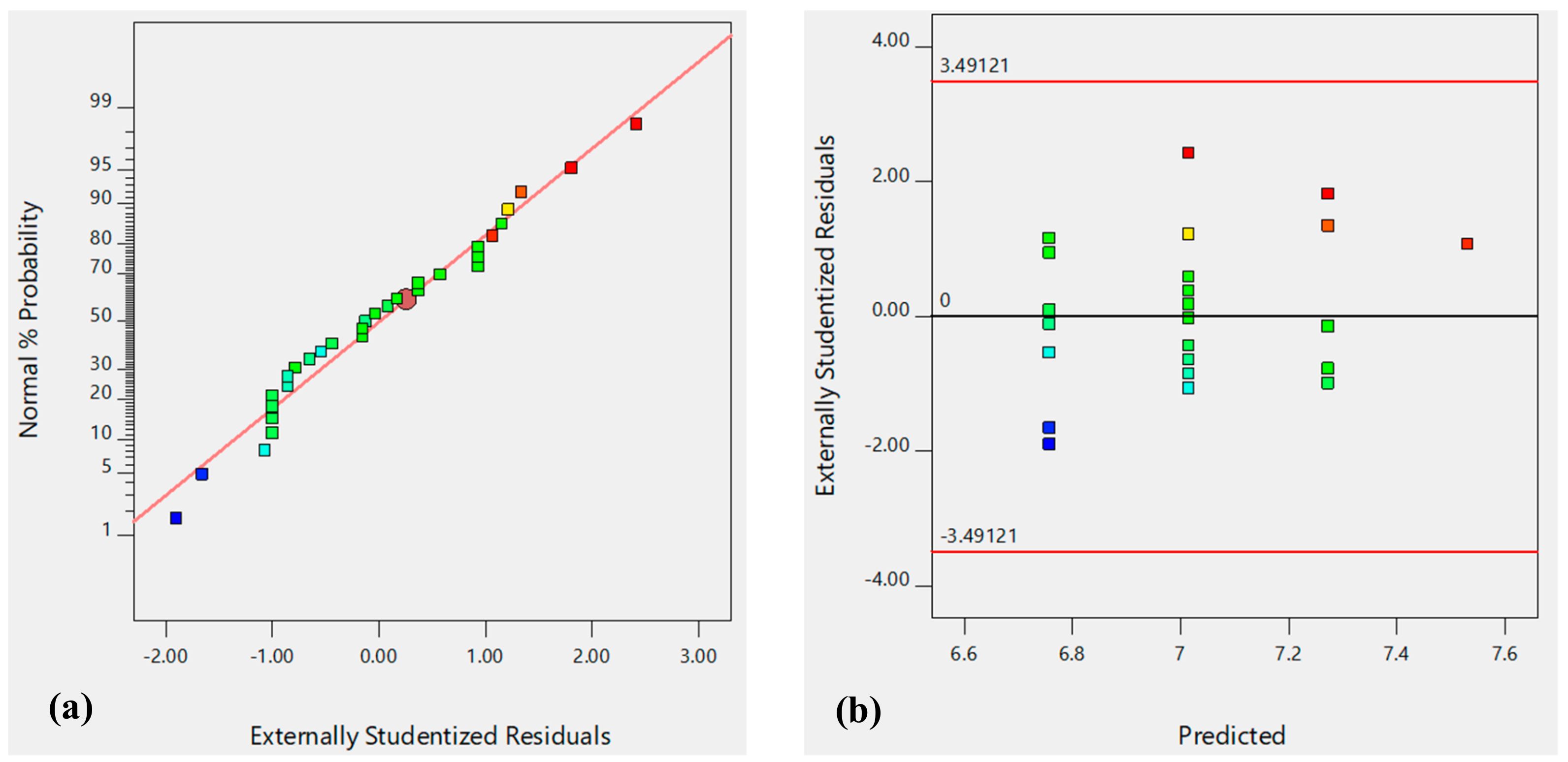
| pHZC | R-sq | R-sq (adj) | R-sq (pred) | F-value | P-value | Residual F | Residual P |
| 0.1711 | 0.1425 | 0.0450 | 5.99 | 0.0207 | 2.45 | 0.3313 | |
| Uncoded equation: | pHZC = +5.85612 + 0.128835pH | ||||||
| Coded equation: | pHZC = +7.02 + 0.2577pH | ||||||
| RMO | R-sq | R-sq (adj) | R-sq (pred) | F-value | P-value | Residual F | Residual P |
| 0.7331 | 0.6920 | 0.6410 | 23.30 | <0.0001 | 5.91 | 0.1548 | |
| Uncoded equation: | RMO = +212.48271 − 10.319pH − 0.326843T − 14.75094t | ||||||
| Coded equation: | RMO = +48.46 − 20.64pH − 16.34T − 7.38t | ||||||
| RMB | R-sq | R-sq (adj) | R-sq (pred) | F-value | P-value | Residual F | Residual P |
| 0.3453 | 0.2478 | 0.3220 | 14.77 | 0.0006 | 7.338.59 | 0.1094 | |
| Uncoded equation: | RMB = +123.11199 − 0.363235T | ||||||
| Coded equation: | RMB = +68.63 − 18.16T | ||||||
| qMO | R-sq | R-sq (adj) | R-sq (pred) | F-value | P-value | Residual F | Residual P |
| 0.7185 | 0.6847 | 0.6301 | 21.27 | <0.0001 | 93.15 | 0.0817 | |
| Uncoded equation: | qMO = +17.08333 − 0.84444pH − 0.026065T − 1.17318t | ||||||
| Coded equation: | qMO = +3.81 − 1.69pH − 1.3T − 0.5866t | ||||||
| qMB | R-sq | R-sq (adj) | R-sq (pred) | F-value | P-value | Residual F | Residual P |
| 0.3467 | 0.3226 | 0.2482 | 14.33 | 0.0008 | 10.45 | 0.2404 | |
| Uncoded equation: | qMB = +9.80868 − 0.029016T | ||||||
| Coded equation: | qMB = +5.46 − 1.45T | ||||||
| Adsorbate | Adsorption Conditions | q (mg/g) | Reference |
|---|---|---|---|
| MB | pH = 6, m = 0.5 g, initial concentration (Ci) = 40 ppm | 34.19 | [37] |
| MB | V/m = 0.625 L/g, Ci = 50 ppm | 27.45 | [38] |
| MB | V/m = 10 L/g, pH = 6, Ci = 100 ppm | 198 | [39] |
| MB | V/m = 1 L/g, pH = 6, Ci = 10 mmol/L | 7 mmol/g | [40] |
| MB | V/m = 0.5 L/g, Ci = 16 ppm | 7.67 | [41] |
| MO | V/m = 2 L/g, pH = 6.5, Ci = 50 ppm | 11 | [42] |
| MB | V/m = 0.4 L/g, pH = 8, Ci = 20 ppm | 7.86 | Current work (sample 29) |
| MO | V/m = 0.4 L/g, pH = 4.5, Ci = 20 ppm | 7.66 | Current work (sample 10) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, M.M.; Da’na, E.; Al-Amer, K.; Hessien, M. Experimental Design Modeling of the Effect of Hexagonal Wurtzite—ZnO Synthesis Conditions on Its Characteristics and Performance as a Cationic and Anionic Adsorbent. Molecules 2019, 24, 3884. https://doi.org/10.3390/molecules24213884
Khalaf MM, Da’na E, Al-Amer K, Hessien M. Experimental Design Modeling of the Effect of Hexagonal Wurtzite—ZnO Synthesis Conditions on Its Characteristics and Performance as a Cationic and Anionic Adsorbent. Molecules. 2019; 24(21):3884. https://doi.org/10.3390/molecules24213884
Chicago/Turabian StyleKhalaf, Mai M., Enshirah Da’na, Kawther Al-Amer, and Manal Hessien. 2019. "Experimental Design Modeling of the Effect of Hexagonal Wurtzite—ZnO Synthesis Conditions on Its Characteristics and Performance as a Cationic and Anionic Adsorbent" Molecules 24, no. 21: 3884. https://doi.org/10.3390/molecules24213884





