Fabrication of Spherical Titania Inverse Opal Structures Using Electro-Hydrodynamic Atomization
Abstract
:1. Introduction
2. Results
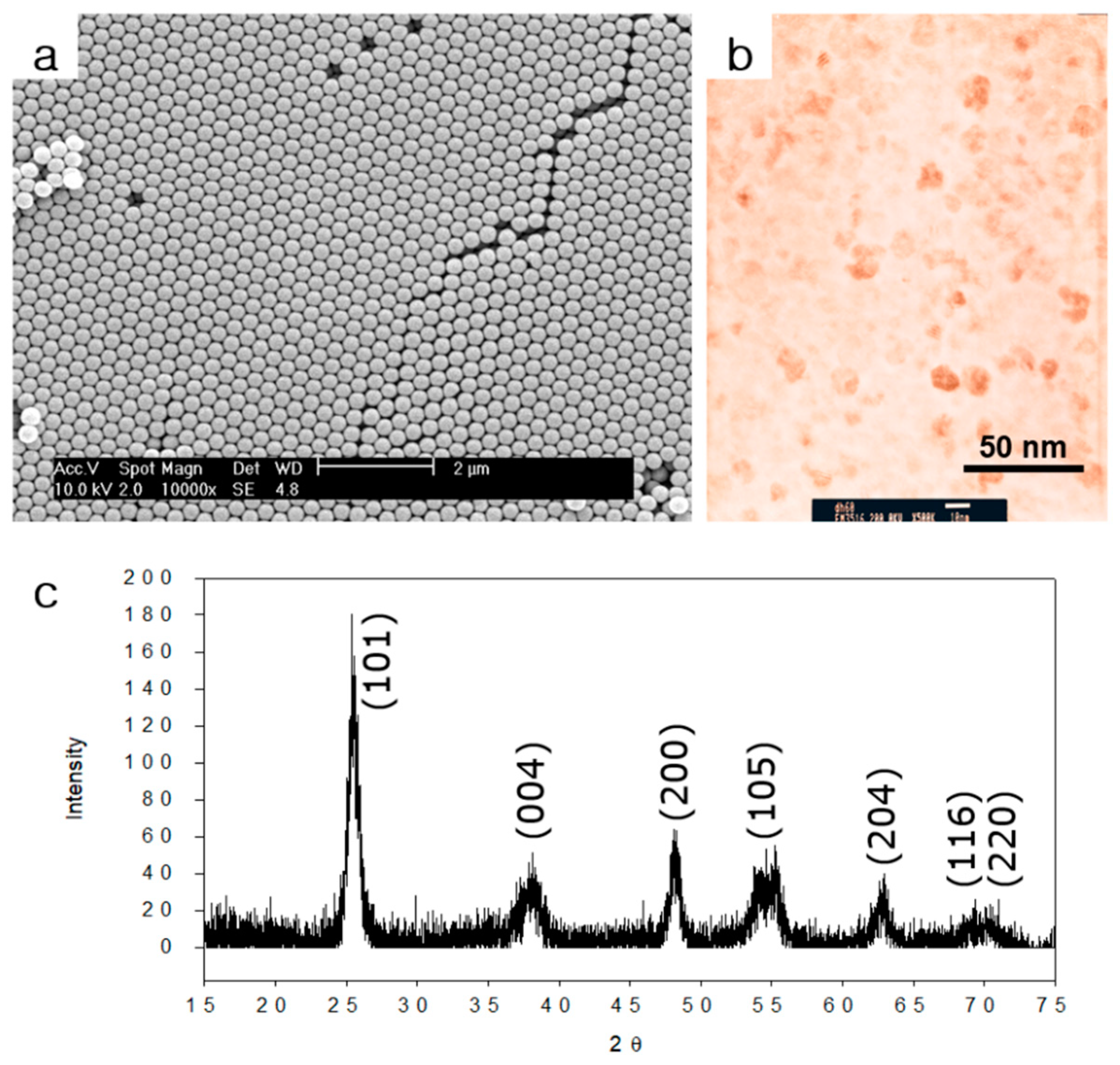
2.1. Characterization of Monodisperse PS/HEMA Nanoparticles and Titania Nanoparticles
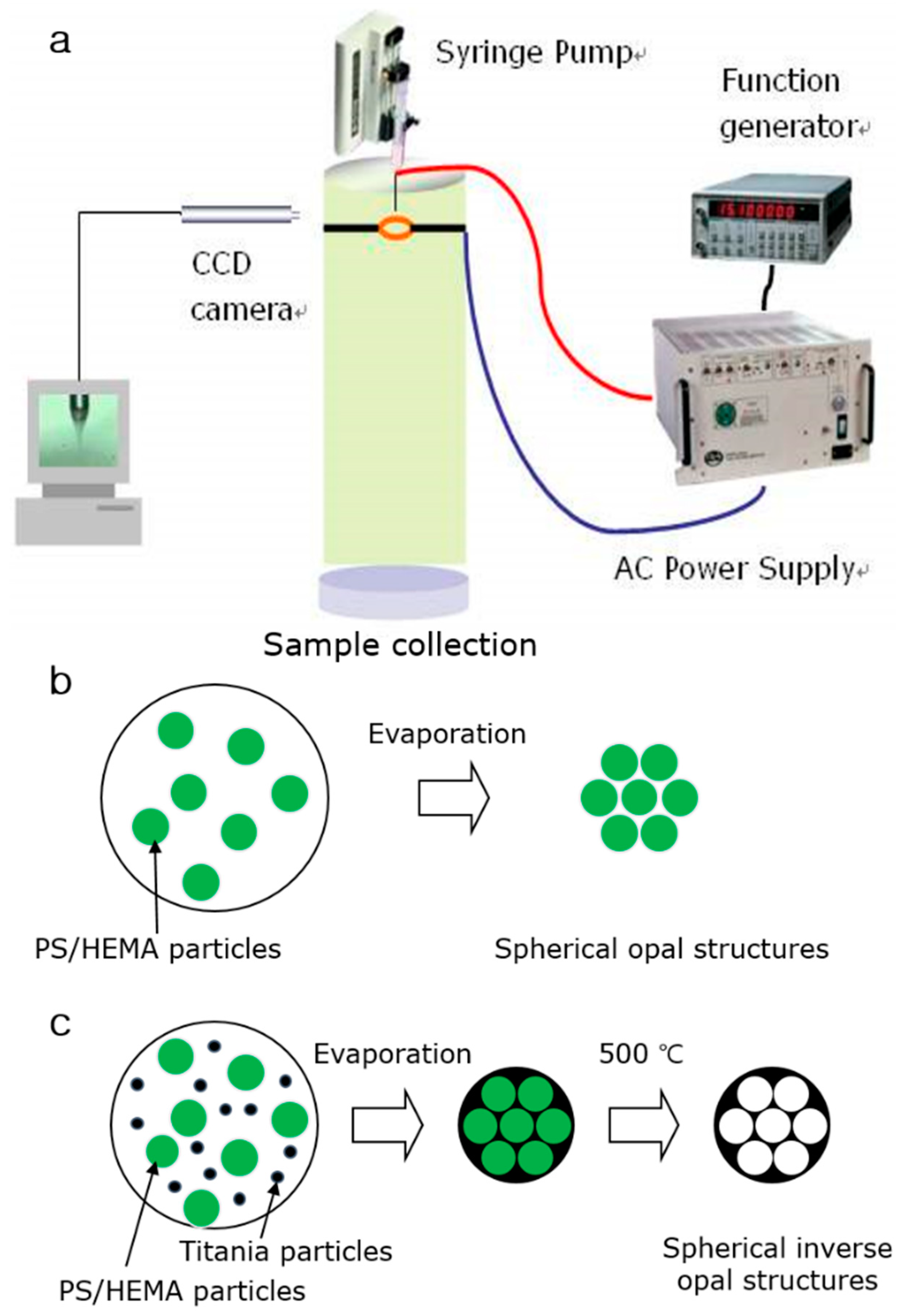
2.2. Preparation of Uniform Aerosol Droplets Using Electro-hydrodynamic Atomization
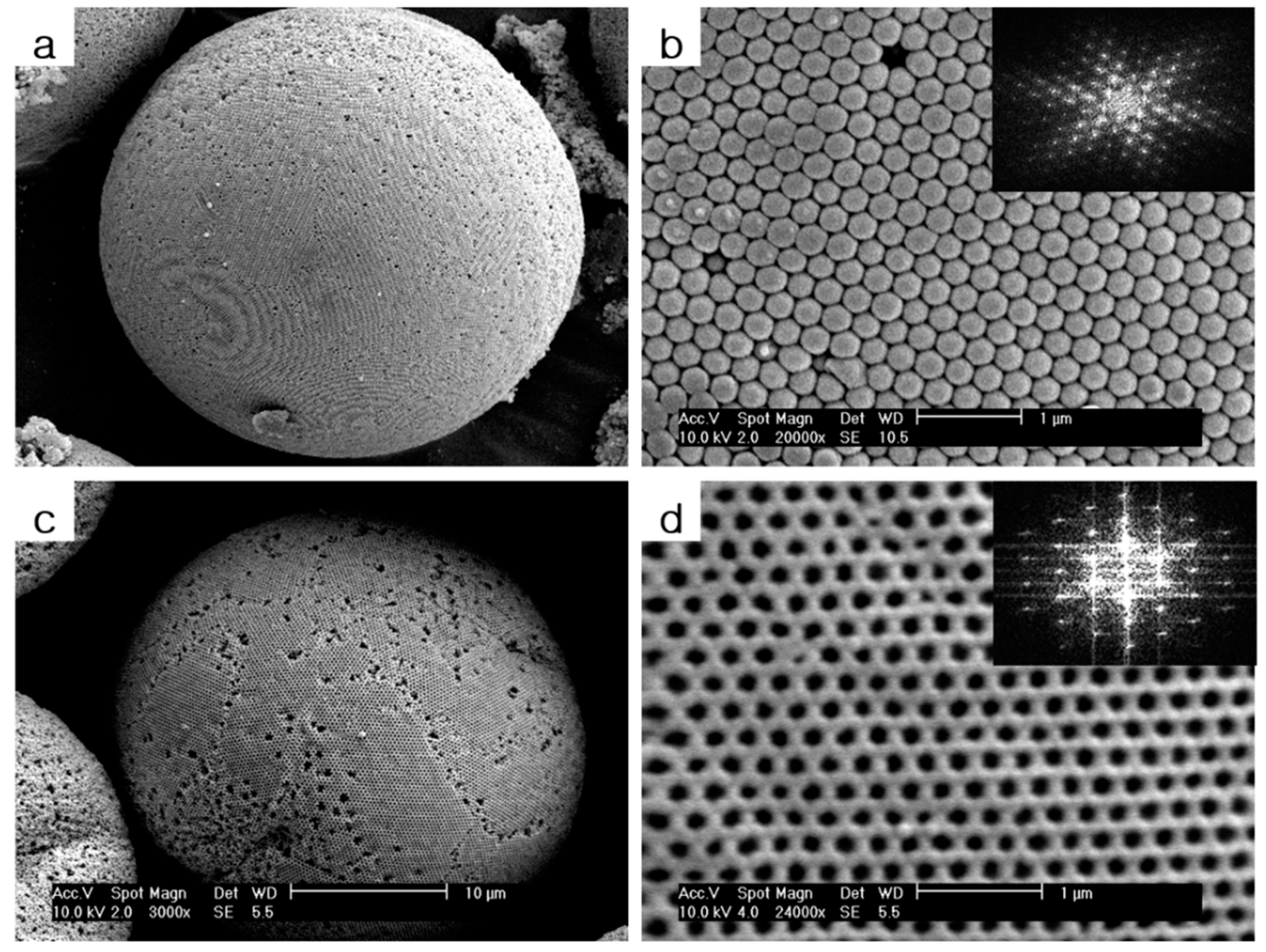
2.3. Fabrication of Spherical PS/HEMA Opal Structures Structures
2.4. Fabrication of Spherical Titania Inverse Opal Structures
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of Monodisperse PS/HEMA Nanoparticles
4.3. Fabrication of Aerosol Droplets Using Electro-hydrodynamic Atomization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yablonovitch, E. Inhibited Spontaneous Emission in Solid-State Physics and Electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef] [PubMed]
- López, C. Materials Aspects of Photonic Crystals. Adv. Mater. 2003, 15, 1679–1704. [Google Scholar] [CrossRef]
- Arsenault, A.; Fleischhaker, F.; Von Freymann, G.; Kitaev, V.; Míguez, H.; Mihi, A.; Tétreault, N.; Vekris, E.; Manners, I.; Aitchison, S.; et al. Perfecting Imperfection—Designer Defects in Colloidal Photonic Crystals. Adv. Mater. 2006, 18, 2779–2785. [Google Scholar] [CrossRef]
- Moon, J.H.; Yang, S. Chemical Aspects of Three-Dimensional Photonic Crystals. Chem. Rev. 2010, 110, 547–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, S.Y.; Yang, S.-M.; Yi, G.-R. Self-assembled colloidal structures for photonics. NPG Asia Mater. 2011, 3, 25–33. [Google Scholar]
- Wang, J.; Zhu, J. Recent advances in spherical photonic crystals: Generation and applications in optics. Eur. Polym. J. 2013, 49, 3420–3433. [Google Scholar] [CrossRef]
- Stein, A.; Li, F.; Denny, N.R. Morphological Control in Colloidal Crystal Templating of Inverse Opals, Hierarchical Structures, and Shaped Particles†. Chem. Mater. 2008, 20, 649–666. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, S.Y.; Yi, G.-R.; Pine, D.J.; Yang, S.-M. Microwave-Assisted Self-Organization of Colloidal Particles in Confining Aqueous Droplets. J. Am. Chem. Soc. 2006, 128, 10897–10904. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Yi, G.-R.; Yang, S.-M.; Park, S.B.; Yi, G.; Yang, S.; Pine, D.J. Electrospray-Assisted Fabrication of Uniform Photonic Balls†. Adv. Mater. 2004, 16, 605–609. [Google Scholar] [CrossRef]
- Hong, S.-H.; Moon, J.H.; Lim, J.-M.; Kim, S.-H.; Yang, S.-M. Fabrication of Spherical Colloidal Crystals Using Electrospray. Langmuir 2005, 21, 10416–10421. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, H.; Chen, A.; Meng, B.; Li, X. Ordered macroporous titania photonic balls by micrometer-scale spherical assembly templating. J. Mater. Chem. 2005, 15, 2551–2556. [Google Scholar] [CrossRef]
- Iskandar, F.; Nandiyanto, A.B.D.; Yun, K.M.; Hogan, C.J.; Okuyama, K.; Biswas, P.; Hogan, C. Enhanced Photocatalytic Performance of Brookite TiO2 Macroporous Particles Prepared by Spray Drying with Colloidal Templating. Adv. Mater. 2007, 19, 1408–1412. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, H.; Jang, S.-Y.; Jo, S.M.; Kim, D.; Seo, Y.; Kim, D.Y. Electrospray Preparation of Hierarchically-structured Mesoporous TiO2 Spheres for Use in Highly Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wu, D.; Li, Q.; Dong, H.; Li, J.; Jiang, K.; Xu, D. Hierarchical TiO2 microspheres: Synthesis, structural control and their applications in dye-sensitized solar cells. RSC Adv. 2012, 2, 11629–11637. [Google Scholar] [CrossRef]
- Chen, D.; Caruso, R.A. Recent progress in the synthesis of spherical titania nanostructures and their applications. Adv. Funct. Mater. 2013, 23, 1356–1374. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Zaleska, A.; Bein, T. Three-Dimensional Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9487–9558. [Google Scholar] [CrossRef] [PubMed]
- Taylor G, I. Disintegration of water drops in an electric field. Proceedings of the Royal Society of London. Series A. Math. Phys. Sci. 1964, 280, 383–397. [Google Scholar]
- Lee, S.Y.; Kim, S.-H.; Hwang, H.; Sim, J.Y.; Yang, S.-M. Controlled Pixelation of Inverse Opaline Structures Towards Reflection-Mode Displays. Adv. Mater. 2014, 26, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.H.; Leite, C.A.P.; Galembeck, F. Elemental Distribution within Single Latex Particles: Determination by Electron Spectroscopy Imaging. Langmuir 1998, 14, 3187–3194. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-M.; Jeong, S. Fabrication of Spherical Titania Inverse Opal Structures Using Electro-Hydrodynamic Atomization. Molecules 2019, 24, 3905. https://doi.org/10.3390/molecules24213905
Lim J-M, Jeong S. Fabrication of Spherical Titania Inverse Opal Structures Using Electro-Hydrodynamic Atomization. Molecules. 2019; 24(21):3905. https://doi.org/10.3390/molecules24213905
Chicago/Turabian StyleLim, Jong-Min, and Sehee Jeong. 2019. "Fabrication of Spherical Titania Inverse Opal Structures Using Electro-Hydrodynamic Atomization" Molecules 24, no. 21: 3905. https://doi.org/10.3390/molecules24213905






