The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment
Abstract
:1. Introduction
2. General Functions and Regulation of MMPs
2.1. The ECM Digestion Processes of MMPs
2.2. The Regulation of MMPs Corresponding to Physiological Processes
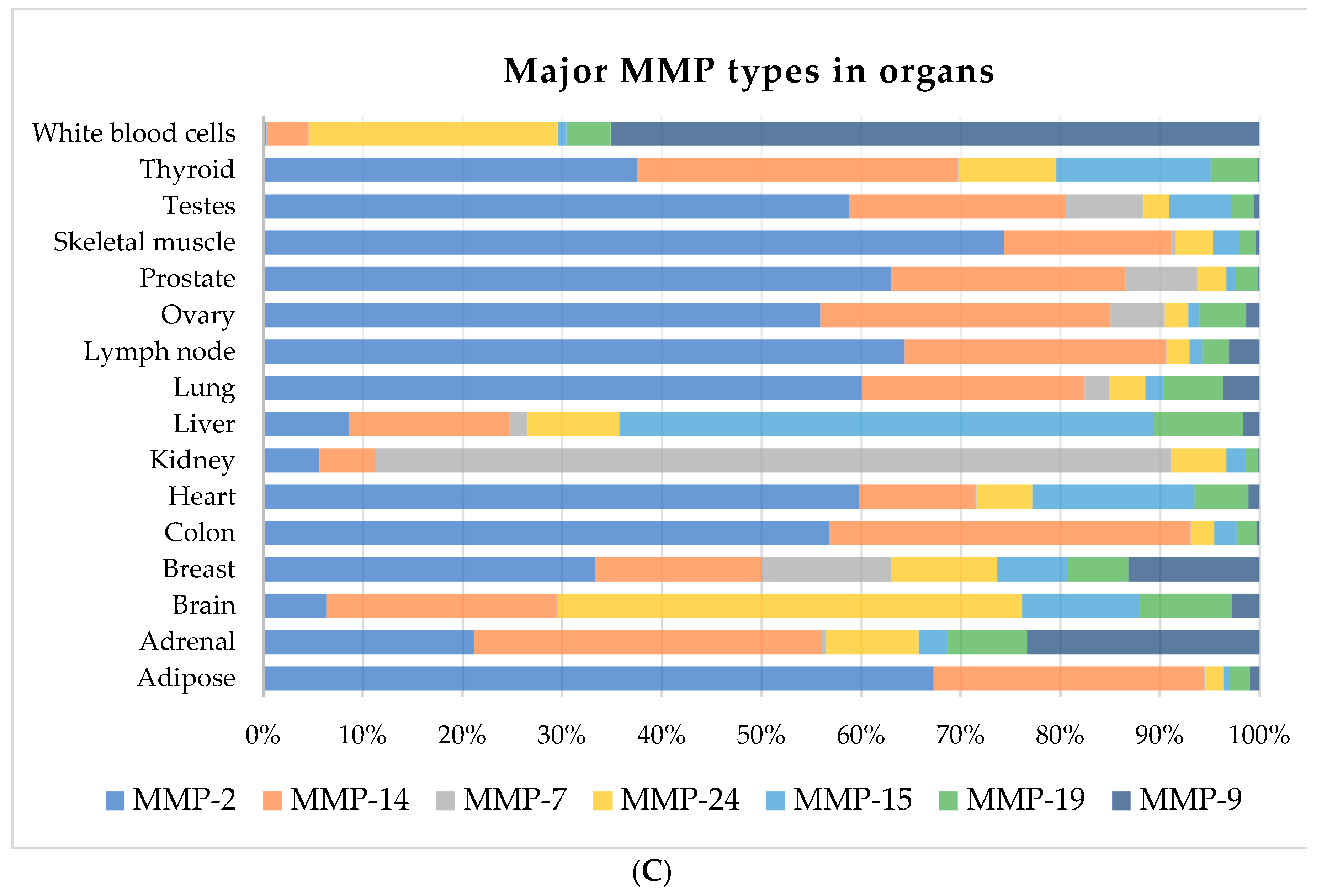
2.3. The Connection of Expression Profile and Organs
3. The Role of ECM Degradation in Fibrosis Treatment
3.1. Rationale for MMPs in Digesting Fibers
3.2. Therapeutic Potential of MMP Inhibition but Not Activation
4. More Functions of MMPs
4.1. Promotion of Cancer Invasiveness
4.2. Macrophage Degradation of the Basal Membrane
4.3. MMPs Treat Stroke or Cardiovascular Diseases
4.4. Central Nervous System (CNS) and the Microenvironment
5. Possible Participating Role of MMPs in Fibrosis
5.1. Immunomodulation or Inflammatory Regulation
5.2. ECM and Vasculature in Angiogenesis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ECM | extracellular matrix |
| MMPs | matrix metalloproteinases |
| MT-MMPs | membrane-type MMPs |
| ADAMs | a disintegrin and metalloproteinases |
| ADAMTs | a disintegrin and metalloproteinases with thrombospondin motifs |
| EGF | epidermal growth factor |
| TSP-1 | type-1 thrombospondin |
| TNF-α | tumor necrosis factor-α |
| TIMPs | tissue inhibitors of metalloproteinases |
| TIMPs | unilateral ureteral obstruction |
| VEGF | vascular epithelial growth factor |
| TLR4 | Toll-like receptor 4 |
| AMPK-α | AMP-activated protein kinase α |
| CCN2/CTGF | cellular communication network factor 2 or connective tissue growth factor |
| AP-1 | activator protein 1 |
| PEA3 | polyoma enhancer activator 3 |
| EGF | epidermal growth factor |
| WBC | white blood cell |
| TGF -β1 | transforming growth factor β1 |
| HIF-1α | hypoxia-inducible factor 1α |
References
- Huntley, G.W. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 2012, 13, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed]
- Lopez-Otin, C.; Hunter, T. The regulatory crosstalk between kinases and proteases in cancer. Nat. Rev. Cancer 2010, 10, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Urbach, C.; Gordon, N.C.; Strickland, I.; Lowne, D.; Joberty-Candotti, C.; May, R.; Herath, A.; Hijnen, D.; Thijs, J.L.; Bruijnzeel-Koomen, C.A.; et al. Combinatorial Screening Identifies Novel Promiscuous Matrix Metalloproteinase Activities that Lead to Inhibition of the Therapeutic Target IL-13. Chem. Biol. 2015, 22, 1442–1452. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. MMP-1: The elder of the family. Int. J. Biochem. Cell Biol. 2005, 37, 283–288. [Google Scholar] [CrossRef]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Active metalloproteases of the A Disintegrin and Metalloprotease (ADAM) family: Biological function and structure. J. Proteome Res. 2011, 10, 17–33. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Fingleton, B. MMPs as therapeutic targets—Still a viable option? Semin. Cell Dev. Biol. 2008, 19, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Manoury, B.; Nenan, S.; Guenon, I.; Lagente, V.; Boichot, E. Influence of early neutrophil depletion on MMPs/TIMP-1 balance in bleomycin-induced lung fibrosis. Int. Immunopharmacol. 2007, 7, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.C.; Markle, L.S.; Vickers, J.L.; Petitt, M.S.; Raimer, S.S.; McNeese, C. The imbalanced expression of matrix metalloproteinases in nephrogenic systemic fibrosis. J. Am. Acad. Dermatol. 2010, 63, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Bramhall, S.R.; Neoptolemos, J.P.; Stamp, G.W.; Lemoine, N.R. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J. Pathol. 1997, 182, 347–355. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, G.Y.; Kim, J.I.; Yoon, M.K.; Kwon, T.K.; Lee, S.J.; Choi, Y.W.; Kang, H.S.; Yoo, Y.H.; Choi, Y.H. Anti-invasive activity of diallyl disulfide through tightening of tight junctions and inhibition of matrix metalloproteinase activities in LNCaP prostate cancer cells. Toxicol. In Vitro 2010, 24, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.P.; Shah, S.V.; Shukla, S.N.; Shah, P.M.; Patel, P.S. Clinical significance of MMP-2 and MMP-9 in patients with oral cancer. Head Neck 2007, 29, 564–572. [Google Scholar] [CrossRef]
- Sutnar, A.; Pesta, M.; Liska, V.; Treska, V.; Skalicky, T.; Kormunda, S.; Topolcan, O.; Cerny, R.; Holubec, L., Jr. Clinical relevance of the expression of mRNA of MMP-7, MMP-9, TIMP-1, TIMP-2 and CEA tissue samples from colorectal liver metastases. Tumour Biol. 2007, 28, 247–252. [Google Scholar] [CrossRef]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef]
- Noel, A.; Gutierrez-Fernandez, A.; Sounni, N.E.; Behrendt, N.; Maquoi, E.; Lund, I.K.; Cal, S.; Hoyer-Hansen, G.; Lopez-Otin, C. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front. Pharmacol. 2012, 3, 140. [Google Scholar] [CrossRef]
- Nishida, Y.; Miyamori, H.; Thompson, E.W.; Takino, T.; Endo, Y.; Sato, H. Activation of matrix metalloproteinase-2 (MMP-2) by membrane type 1 matrix metalloproteinase through an artificial receptor for proMMP-2 generates active MMP-2. Cancer Res. 2008, 68, 9096–9104. [Google Scholar] [CrossRef]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland. Biol. Neoplasia 2010, 15, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hemmann, S.; Graf, J.; Roderfeld, M.; Roeb, E. Expression of MMPs and TIMPs in liver fibrosis—A systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 2007, 46, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Matrisian, L.M.; Kells, A.F.; Pearson, R.; Yuan, Z.Y.; Navre, M. Mutational analysis of the transin (rat stromelysin) autoinhibitor region demonstrates a role for residues surrounding the “cysteine switch”. J. Biol. Chem. 1991, 266, 1584–1590. [Google Scholar]
- Eguchi, T.; Kubota, S.; Kawata, K.; Mukudai, Y.; Uehara, J.; Ohgawara, T.; Ibaragi, S.; Sasaki, A.; Kuboki, T.; Takigawa, M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol. Cell. Biol. 2008, 28, 2391–2413. [Google Scholar] [CrossRef]
- Ali, M.A.; Chow, A.K.; Kandasamy, A.D.; Fan, X.; West, L.J.; Crawford, B.D.; Simmen, T.; Schulz, R. Mechanisms of cytosolic targeting of matrix metalloproteinase-2. J. Cell. Physiol. 2012, 227, 3397–3404. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, L.; Wang, Z.; Cai, Y.; Xu, Q.; Chen, P. Hypoxia Activates Src and Promotes Endocytosis Which Decreases MMP-2 Activity and Aggravates Renal Interstitial Fibrosis. Int. J. Mol. Sci. 2018, 19, 581. [Google Scholar] [CrossRef]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef]
- Fischer, T.; Riedl, R. Inhibitory Antibodies Designed for Matrix Metalloproteinase Modulation. Molecules 2019, 24, 2265. [Google Scholar] [CrossRef]
- Peralta, F.A.; Huidobro-Toro, J.P. Zinc as Allosteric Ion Channel Modulator: Ionotropic Receptors as Metalloproteins. Int. J. Mol. Sci. 2016, 17, 1059. [Google Scholar] [CrossRef]
- Pelmenschikov, V.; Siegbahn, P.E. Catalytic mechanism of matrix metalloproteinases: Two-layered ONIOM study. Inorg. Chem. 2002, 41, 5659–5666. [Google Scholar] [CrossRef] [PubMed]
- Manzetti, S.; McCulloch, D.R.; Herington, A.C.; van der Spoel, D. Modeling of enzyme-substrate complexes for the metalloproteases MMP-3, ADAM-9 and ADAM-10. J. Comput. Aided Mol. Des. 2003, 17, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Massova, I.; Kotra, L.P.; Fridman, R.; Mobashery, S. Matrix metalloproteinases: Structures, evolution, and diversification. FASEB J. 1998, 12, 1075–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, S.J.; Pereira, H.M.; Jacchieri, S.; Brentani, R.R. Collagen/collagenase interaction: Does the enzyme mimic the conformation of its own substrate? FASEB J. 1996, 10, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, W. A helping hand for collagenases: The haemopexin-like domain. Structure 1995, 3, 527–530. [Google Scholar] [CrossRef]
- Nagase, H. Substrate Specificity of MMPs. In Matrix Metalloproteinase Inhibitors in Cancer Therapy; Clendeninn, N.J., Appelt, K., Eds.; Humana Press: Totowa, NJ, USA, 2001; pp. 39–66. [Google Scholar]
- Gomis-Ruth, F.X.; Gohlke, U.; Betz, M.; Knauper, V.; Murphy, G.; Lopez-Otin, C.; Bode, W. The helping hand of collagenase-3 (MMP-13): 2.7 A crystal structure of its C-terminal haemopexin-like domain. J. Mol. Biol. 1996, 264, 556–566. [Google Scholar] [CrossRef]
- Yan, C.; Boyd, D.D. Regulation of matrix metalloproteinase gene expression. J. Cell. Physiol. 2007, 211, 19–26. [Google Scholar] [CrossRef]
- Hattori, N.; Mochizuki, S.; Kishi, K.; Nakajima, T.; Takaishi, H.; D’Armiento, J.; Okada, Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am. J. Pathol. 2009, 175, 533–546. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Chung, J.H. Epidermal growth factor-induced matrix metalloproteinase-1 expression is negatively regulated by p38 MAPK in human skin fibroblasts. J. Dermatol. Sci. 2011, 64, 134–141. [Google Scholar] [CrossRef]
- Lachowski, D.; Cortes, E.; Rice, A.; Pinato, D.; Rombouts, K.; Hernandez, A.D.R. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis. Sci. Rep. 2019, 9, 7299. [Google Scholar] [CrossRef] [PubMed]
- Van Wart, H.E.; Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, D.E.; Alonso, D.F.; Yoshiji, H.; Thorgeirsson, U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997, 74, 111–122. [Google Scholar] [PubMed]
- Tandara, A.A.; Mustoe, T.A. MMP- and TIMP-secretion by human cutaneous keratinocytes and fibroblasts—Impact of coculture and hydration. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Nkyimbeng, T.; Ruppert, C.; Shiomi, T.; Dahal, B.; Lang, G.; Seeger, W.; Okada, Y.; D’Armiento, J.; Gunther, A. Pivotal role of matrix metalloproteinase 13 in extracellular matrix turnover in idiopathic pulmonary fibrosis. PLoS ONE 2013, 8, e73279. [Google Scholar] [CrossRef]
- Uchinami, H.; Seki, E.; Brenner, D.A.; D’Armiento, J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology 2006, 44, 420–429. [Google Scholar] [CrossRef]
- Pardo, A.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Rosas, I.O.; Richards, T.J.; Konishi, K.; Zhang, Y.; Gibson, K.; Lokshin, A.E.; Lindell, K.O.; Cisneros, J.; Macdonald, S.D.; Pardo, A.; et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008, 5, e93. [Google Scholar] [CrossRef] [Green Version]
- Sokai, A.; Handa, T.; Tanizawa, K.; Oga, T.; Uno, K.; Tsuruyama, T.; Kubo, T.; Ikezoe, K.; Nakatsuka, Y.; Tanimura, K.; et al. Matrix metalloproteinase-10: A novel biomarker for idiopathic pulmonary fibrosis. Respir. Res. 2015, 16, 120. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Ikeda, T.; Gomi, H.; Nakao, S.; Suzuki, T.; Itohara, S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J. Biol. Chem. 1997, 272, 22389–22392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendas, A.M.; Folgueras, A.R.; Llano, E.; Caterina, J.; Frerard, F.; Rodriguez, F.; Astudillo, A.; Noel, A.; Birkedal-Hansen, H.; Lopez-Otin, C. Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol. Cell. Biol. 2004, 24, 5304–5313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, R.; Lefebvre, O.; Noel, A.; Fahime, M.E.; Chenard, M.P.; Wendling, C.; Kebers, F.; LeMeur, M.; Dierich, A.; Foidart, J.M.; et al. In vivo evidence that the stromelysin-3 metalloproteinase contributes in a paracrine manner to epithelial cell malignancy. J. Cell Biol. 1998, 140, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc. Am. Thorac. Soc. 2006, 3, 383–388. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choeng, H.C.; Ahn, C.; Cho, S.H. Early and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary fibrosis. Yonsei Med. J. 2009, 50, 68–77. [Google Scholar] [CrossRef]
- Corbel, M.; Boichot, E.; Lagente, V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz. J. Med. Biol. Res. 2000, 33, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Pardo, A.; Barrios, R.; Maldonado, V.; Melendez, J.; Perez, J.; Ruiz, V.; Segura-Valdez, L.; Sznajder, J.I.; Selman, M. Gelatinases A and B are up-regulated in rat lungs by subacute hyperoxia: Pathogenetic implications. Am. J. Pathol. 1998, 153, 833–844. [Google Scholar] [CrossRef]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Stevens, L.J.; Page-McCaw, A. A secreted MMP is required for reepithelialization during wound healing. Mol. Biol. Cell 2012, 23, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, I.; Arai, M.; Wada, N.; Maruyama, K. Gene expression of MMPs and TIMPs in the process of hepatic fibrosis. Nihon Rinsho 1993, 51, 428–434. [Google Scholar] [PubMed]
- Laszlo, A.; Sohar, I.; Gyurkovits, K. Activity of the lysosomal cysteine proteinases (cathepsin B,H,L) and a metalloproteinase (MMP-7-ase) in the serum of cystic fibrosis homozygous children. Acta Paediatr. Hung. 1987, 28, 175–178. [Google Scholar] [PubMed]
- Takahara, T.; Furui, K.; Funaki, J.; Nakayama, Y.; Itoh, H.; Miyabayashi, C.; Sato, H.; Seiki, M.; Ooshima, A.; Watanabe, A. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995, 21, 787–795. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Iturria, C.; Vadillo-Ortega, F.; Ovalle, C.; Montano, M. Changes in matrix metalloproteinases during the evolution of interstitial renal fibrosis in a rat experimental model. Pathobiology 1998, 66, 196–204. [Google Scholar] [CrossRef]
- Hayashi, T.; Stetler-Stevenson, W.G.; Fleming, M.V.; Fishback, N.; Koss, M.N.; Liotta, L.A.; Ferrans, V.J.; Travis, W.D. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am. J. Pathol. 1996, 149, 1241–1256. [Google Scholar]
- Lafuma, C.; El Nabout, R.A.; Crechet, F.; Hovnanian, A.; Martin, M. Expression of 72-kDa gelatinase (MMP-2), collagenase (MMP-1), and tissue metalloproteinase inhibitor (TIMP) in primary pig skin fibroblast cultures derived from radiation-induced skin fibrosis. J. Investig. Dermatol. 1994, 102, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qiao, X.; Tan, T.K.; Zhao, H.; Zhang, Y.; Liu, L.; Zhang, J.; Wang, L.; Cao, Q.; Wang, Y.; et al. Matrix metalloproteinase 9-dependent Notch signaling contributes to kidney fibrosis through peritubular endothelial-mesenchymal transition. Nephrol. Dial. Transplant. 2017, 32, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Arthur, M.J. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G245–G249. [Google Scholar] [CrossRef]
- Henry, M.T.; McMahon, K.; Mackarel, A.J.; Prikk, K.; Sorsa, T.; Maisi, P.; Sepper, R.; Fitzgerald, M.X.; O’Connor, C.M. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-1 in sarcoidosis and IPF. Eur. Respir. J. 2002, 20, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Hovell, C.J.; Pawley, S.; Hutchings, M.I.; Arthur, M.J.; Iredale, J.P.; Benyon, R.C. Expression of matrix metalloproteinase-2 and -14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int. 2004, 24, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Nag, S.; Bardhan, S.; Acharya, D.; Paul, R.; Dey, R.; Ghosh, M.; Saha, I. The role of long-term doxycycline in patients of idiopathic pulmonaryfibrosis: The results of an open prospective trial. Lung India 2009, 26, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bhattacharya, P.; Paul, S.; Paul, R.; Swarnakar, S. An alternative therapy for idiopathic pulmonary fibrosis by doxycycline through matrix metalloproteinase inhibition. Lung India 2011, 28, 174–179. [Google Scholar] [PubMed]
- England, K.A.; Price, A.P.; Tram, K.V.; Shapiro, S.D.; Blazar, B.R.; Panoskaltsis-Mortari, A. Evidence for early fibrosis and increased airway resistance in bone marrow transplant recipient mice deficient in MMP12. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L519–L526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.R.; Cho, S.J.; Lee, C.G.; Homer, R.J.; Elias, J.A. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J. Biol. Chem. 2007, 282, 7723–7732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanone, S.; Zheng, T.; Zhu, Z.; Liu, W.; Lee, C.G.; Ma, B.; Chen, Q.; Homer, R.J.; Wang, J.; Rabach, L.A.; et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J. Clin. Investig. 2002, 110, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef] [Green Version]
- Margulis, A.; Nocka, K.H.; Wood, N.L.; Wolf, S.F.; Goldman, S.J.; Kasaian, M.T. MMP dependence of fibroblast contraction and collagen production induced by human mast cell activation in a three-dimensional collagen lattice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L236–L247. [Google Scholar] [CrossRef]
- Malek, V.; Gaikwad, A.B. Telmisartan and thiorphan combination treatment attenuates fibrosis and apoptosis in preventing diabetic cardiomyopathy. Cardiovasc. Res. 2019, 115, 373–384. [Google Scholar] [CrossRef]
- Corbel, M.; Caulet-Maugendre, S.; Germain, N.; Molet, S.; Lagente, V.; Boichot, E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J. Pathol. 2001, 193, 538–545. [Google Scholar] [CrossRef]
- George, J.; Tsutsumi, M.; Tsuchishima, M. MMP-13 deletion decreases profibrogenic molecules and attenuates N-nitrosodimethylamine-induced liver injury and fibrosis in mice. J. Cell. Mol. Med. 2017, 21, 3821–3835. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T. Matrix metalloproteinase inhibition and the prevention of heart failure. Trends Cardiovasc. Med. 2001, 11, 202–205. [Google Scholar] [CrossRef]
- Fujita, M.; Ye, Q.; Ouchi, H.; Harada, E.; Inoshima, I.; Kuwano, K.; Nakanishi, Y. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob. Agents Chemother. 2006, 50, 739–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, Y.; Kunihiro, S.; Sato, S.; Yoshioka, K.; Hara, Y.; Kanai, K.; Hoshi, F.; Itoh, N.; Higuchi, S. Doxycycline attenuates isoproterenol-induced myocardial fibrosis and matrix metalloproteinase activity in rats. Biol. Pharm. Bull. 2009, 32, 1678–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, R.J.; Fattman, C.L.; Niehouse, L.M.; Tobolewski, J.M.; Hanford, L.E.; Li, Q.; Monzon, F.A.; Parks, W.C.; Oury, T.D. Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2006, 35, 289–297. [Google Scholar] [CrossRef]
- de Meijer, V.E.; Sverdlov, D.Y.; Popov, Y.; Le, H.D.; Meisel, J.A.; Nose, V.; Schuppan, D.; Puder, M. Broad-spectrum matrix metalloproteinase inhibition curbs inflammation and liver injury but aggravates experimental liver fibrosis in mice. PLoS ONE 2010, 5, e11256. [Google Scholar] [CrossRef] [Green Version]
- Pulli, B.; Ali, M.; Iwamoto, Y.; Zeller, M.W.; Schob, S.; Linnoila, J.J.; Chen, J.W. Myeloperoxidase-Hepatocyte-Stellate Cell Cross Talk Promotes Hepatocyte Injury and Fibrosis in Experimental Nonalcoholic Steatohepatitis. Antioxid. Redox Signal. 2015, 23, 1255–1269. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhang, J.; Telljohann, R.; Jiang, L.; Wu, J.; Monticone, R.E.; Kapoor, K.; Talan, M.; Lakatta, E.G. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension 2012, 60, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, P.; Peterson, J.T.; Subramanian, V.; Singh, M.; Singh, K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol. Cell. Biochem. 2009, 322, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Parks, W.C.; Wilson, C.L.; Lopez-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Jia, M.X.; Wang, J.H.; Lu, J.L.; Deng, J.; Tang, J.X.; Liu, C. Association of MMP9-1562C/T and MMP13-77A/G Polymorphisms with Non-Small Cell Lung Cancer in Southern Chinese Population. Biomolecules 2019, 9, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hart, E.; Shchurin, A.; Hoover-Plow, J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J. Clin. Investig. 2008, 118, 3012–3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, M.Y.; Birkland, T.P.; Howe, J.D.; Rowan, A.D.; Fidock, M.; Parks, W.C.; Gavrilovic, J. Macrophage migration and invasion is regulated by MMP10 expression. PLoS ONE 2013, 8, e63555. [Google Scholar] [CrossRef] [Green Version]
- Shipley, J.M.; Wesselschmidt, R.L.; Kobayashi, D.K.; Ley, T.J.; Shapiro, S.D. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 3942–3946. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Masson, V.; de la Ballina, L.R.; Munaut, C.; Wielockx, B.; Jost, M.; Maillard, C.; Blacher, S.; Bajou, K.; Itoh, T.; Itohara, S.; et al. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J. 2005, 19, 234–236. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, T.Y.; Tsao, S.M.; Yeh, C.B.; Yang, S.F. Matrix metalloproteinases in pneumonia. Clin. Chim. Acta 2014, 433, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, G.; Quchani, S.H.; Sahraian, M.A.; Abolhassani, F.; Gilani, M.A.S.; Tarzjani, M.D.; Atoof, F. Leukocyte Gene Expression and Plasma Concentration in Multiple Sclerosis: Alteration of Transforming Growth Factor-betas, Claudin-11, and Matrix Metalloproteinase-2. Cell. Mol. Neurobiol. 2016, 36, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Amorosa, L.F.; Coyle, S.M.; Macor, M.A.; Lubitz, S.E.; Carson, J.L.; Birnbaum, M.J.; Lee, L.Y.; Haimovich, B. Proteolytic Cleavage of AMPKalpha and Intracellular MMP9 Expression Are Both Required for TLR4-Mediated mTORC1 Activation and HIF-1alpha Expression in Leukocytes. J. Immunol. 2015, 195, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Park, J.S.; Choi, I.Y.; Kim, W.K.; Kim, H.S. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J. Neurochem. 2008, 106, 770–780. [Google Scholar] [CrossRef]
- Berg, G.; Barchuk, M.; Miksztowicz, V. Behavior of Metalloproteinases in Adipose Tissue, Liver and Arterial Wall: An Update of Extracellular Matrix Remodeling. Cells 2019, 8, 158. [Google Scholar] [CrossRef] [Green Version]
- Javaid, M.A.; Abdallah, M.N.; Ahmed, A.S.; Sheikh, Z. Matrix metalloproteinases and their pathological upregulation in multiple sclerosis: An overview. Acta Neurol. Belg. 2013, 113, 381–390. [Google Scholar] [CrossRef]
- Hansmann, F.; Zhang, N.; Herder, V.; Leitzen, E.; Baumgartner, W. Delayed Astrogliosis Associated with Reduced M1 Microglia Activation in Matrix Metalloproteinase 12 Knockout Mice during Theiler’s Murine Encephalomyelitis. Int. J. Mol. Sci. 2019, 20, 1702. [Google Scholar] [CrossRef] [Green Version]
- Vedam-Mai, V.; Yachnis, A.; Ullman, M.; Javedan, S.P.; Okun, M.S. Postmortem observation of collagenous lead tip region fibrosis as a rare complication of DBS. Mov. Disord. 2012, 27, 565–569. [Google Scholar] [CrossRef]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef]
- Shimoda, M. Extracellular vesicle-associated MMPs: A modulator of the tissue microenvironment. Adv. Clin. Chem. 2019, 88, 35–66. [Google Scholar] [PubMed]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Koeck, E.S.; Iordanskaia, T.; Sevilla, S.; Ferrante, S.C.; Hubal, M.J.; Freishtat, R.J.; Nadler, E.P. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: A novel paradigm for obesity-related liver disease. J. Surg. Res. 2014, 192, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hakulinen, J.; Sankkila, L.; Sugiyama, N.; Lehti, K.; Keski-Oja, J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J. Cell. Biochem. 2008, 105, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Han, K.Y.; Dugas-Ford, J.; Seiki, M.; Chang, J.H.; Azar, D.T. Evidence for the Involvement of MMP14 in MMP2 Processing and Recruitment in Exosomes of Corneal Fibroblasts. Invest. Ophthalmol. Vis. Sci. 2015, 56, 5323–5329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalfe, P.D.; Wang, J.; Jiao, H.; Huang, Y.; Hori, K.; Moore, R.B.; Tredget, E.E. Bladder outlet obstruction: Progression from inflammation to fibrosis. BJU Int. 2010, 106, 1686–1694. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Daniels, C.E.; Lasky, J.A.; Limper, A.H.; Mieras, K.; Gabor, E.; Schroeder, D.R. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am. J. Respir. Crit. Care Med. 2010, 181, 604–610. [Google Scholar] [CrossRef]
- Boettcher, E.; Csako, G.; Pucino, F.; Wesley, R.; Loomba, R. Meta-analysis: Pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2012, 35, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Anstrom, K.J.; King, T.E., Jr.; Lasky, J.A.; Martinez, F.J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar] [CrossRef]
- Rafii, R.; Juarez, M.M.; Albertson, T.E.; Chan, A.L. A review of current and novel therapies for idiopathic pulmonary fibrosis. J. Thorac. Dis. 2013, 5, 48–73. [Google Scholar] [PubMed]
- Ahluwalia, N.; Shea, B.S.; Tager, A.M. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am. J. Respir. Crit. Care Med. 2014, 190, 867–878. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.; Richter, A.G.; O’Kane, C.; McAuley, D.F.; Thickett, D.R. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur. Respir. J. 2009, 33, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, C.M.; Dolgonos, L.; Zemans, R.L.; Young, S.K.; Robertson, J.; Briones, N.; Suzuki, T.; Campbell, M.N.; Gauldie, J.; Radisky, D.C.; et al. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am. J. Pathol. 2011, 179, 1733–1745. [Google Scholar] [CrossRef]
- Willems, S.; Verleden, S.E.; Vanaudenaerde, B.M.; Wynants, M.; Dooms, C.; Yserbyt, J.; Somers, J.; Verbeken, E.K.; Verleden, G.M.; Wuyts, W.A. Multiplex protein profiling of bronchoalveolar lavage in idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. Ann. Thorac. Med. 2013, 8, 38–45. [Google Scholar]
- Yu, G.; Kovkarova-Naumovski, E.; Jara, P.; Parwani, A.; Kass, D.; Ruiz, V.; Lopez-Otin, C.; Rosas, I.O.; Gibson, K.F.; Cabrera, S.; et al. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am. J. Respir. Crit. Care Med. 2012, 186, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Rohani, M.G.; Dimitrova, E.; Beppu, A.; Wang, Y.; Jefferies, C.A.; Parks, W.C. Macrophage MMP10 Regulates TLR7-Mediated Tolerance. Front. Immunol. 2018, 9, 2817. [Google Scholar] [CrossRef]
- Kriz, W.; Kaissling, B.; Le Hir, M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: Fact or fantasy? J. Clin. Investig. 2011, 121, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.L.; Zhu, R.T.; Sun, Y.L. Epithelial-mesenchymal transition in liver fibrosis. Biomed. Rep. 2016, 4, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.M.; Su, H.L.; Li, C.; Lin, S.Z.; Yen, S.Y.; Huang, M.H.; Ho, L.I.; Chiou, T.W.; Harn, H.J. The Role of Butylidenephthalide in Targeting the Microenvironment Which Contributes to Liver Fibrosis Amelioration. Front. Pharmacol. 2016, 7, 112. [Google Scholar] [CrossRef] [Green Version]
- Marmai, C.; Sutherland, R.E.; Kim, K.K.; Dolganov, G.M.; Fang, X.; Kim, S.S.; Jiang, S.; Golden, J.A.; Hoopes, C.W.; Matthay, M.A.; et al. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L71–L78. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.Y.; Huang, T.P.; Yang, W.C.; Chen, Z.P.; Yang, A.H.; Mu, W.; Nikolic-Paterson, D.J.; Atkins, R.C.; Lan, H.Y. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998, 54, 864–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, S.H. Genesis of the myofibroblast in lung injury and fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef]
- Tian, L.; He, L.S.; Soni, B.; Shang, H.T. Myofibroblasts and their resistance to apoptosis: A possible mechanism of osteoradionecrosis. Clin. Cosmet. Investig. Dent. 2012, 4, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Strand, S.; Vollmer, P.; van den Abeelen, L.; Gottfried, D.; Alla, V.; Heid, H.; Kuball, J.; Theobald, M.; Galle, P.R.; Strand, D. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene 2004, 23, 3732–3736. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Miyake, M.; Lawton, A.; Goodison, S.; Rosser, C.J. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer 2014, 14, 310. [Google Scholar] [CrossRef] [Green Version]
- Seeland, U.; Haeuseler, C.; Hinrichs, R.; Rosenkranz, S.; Pfitzner, T.; Scharffetter-Kochanek, K.; Bohm, M. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur. J. Clin. Investig. 2002, 32, 295–303. [Google Scholar] [CrossRef]
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1beta or TNF-alpha Release from Human Hepatic Stellate Cells. PLoS ONE 2016, 11, e0153118. [Google Scholar] [CrossRef]
- Zheng, H.; Takahashi, H.; Murai, Y.; Cui, Z.; Nomoto, K.; Niwa, H.; Tsuneyama, K.; Takano, Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer. Res. 2006, 26, 3579–3583. [Google Scholar]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 20262–20267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, S.; Fu, A.A.; Misra, K.D.; Shergill, U.M.; Leof, E.B.; Mukhopadhyay, D. Hypoxia-induced phenotypic switch of fibroblasts to myofibroblasts through a matrix metalloproteinase 2/tissue inhibitor of metalloproteinase-mediated pathway: Implications for venous neointimal hyperplasia in hemodialysis access. J. Vasc. Interv. Radiol. 2010, 21, 896–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhard, U.; Huesgen, P.F.; Schilling, O.; Bellac, C.L.; Butler, G.S.; Cox, J.H.; Dufour, A.; Goebeler, V.; Kappelhoff, R.; Keller, U.A.D.; et al. Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol. 2016, 49, 37–60. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [Green Version]

| Compound Name | Description | Effects | CAS Number | Refs |
|---|---|---|---|---|
| Batimastat | A broad-spectrum MMP inhibitor | Inhibit pulmonary fibrosis | 130370-60-4 | [81] |
| CL 82198 hydrochloride | A selective inhibitor of MMP-13 | Blocks liver fibrosis | 307002-71-7 | [82] |
| CP 471474 | An MMP inhibitor | Inhibit collagen in myocardial fibrosis | 210755-45-6 | [83] |
| Doxycycline Hyclate | An antimicrobial tetracycline that acts as an inhibitor of MMP-1, MMP-8 and MMP-9 | Attenuated pulmonary/myocardial fibrosis | 24390-14-5 | [84,85] [74] |
| Reduced parameters in IPF patients | ||||
| GM 6001 | A cell permeable MMP and fibroblast collagenase inhibitor | Reduced pulmonary inflammation and fibrosis | 142880-36-2 | [86] |
| Marimastat | A broad-spectrum MMP inhibitor and selective TACE inhibitor | Aggravates liver fibrosis | 154039-60-8 | [87,88] |
| PD166793 | A potent MMP-2, MMP-3, and MMP-13 inhibitor | Retardation of age-associated arterial fibrosis | 199850-67-4 | [89] [90] |
| Reduced myocardial fibrosis | ||||
| Thiorphan (DL) | An enkephalinase and metalloproteinase inhibitor | Reduced myocardial fibrosis | 76721-89-6 | [80] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, H.-M.; Chen, Y.-S.; Harn, H.-J. The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment. Molecules 2019, 24, 4188. https://doi.org/10.3390/molecules24224188
Chuang H-M, Chen Y-S, Harn H-J. The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment. Molecules. 2019; 24(22):4188. https://doi.org/10.3390/molecules24224188
Chicago/Turabian StyleChuang, Hong-Meng, Yu-Shuan Chen, and Horng-Jyh Harn. 2019. "The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment" Molecules 24, no. 22: 4188. https://doi.org/10.3390/molecules24224188






