Effects of Different Colored LEDs on the Enhancement of Biologically Active Ingredients in Callus Cultures of Gynura procumbens (Lour.) Merr.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Callus Induction
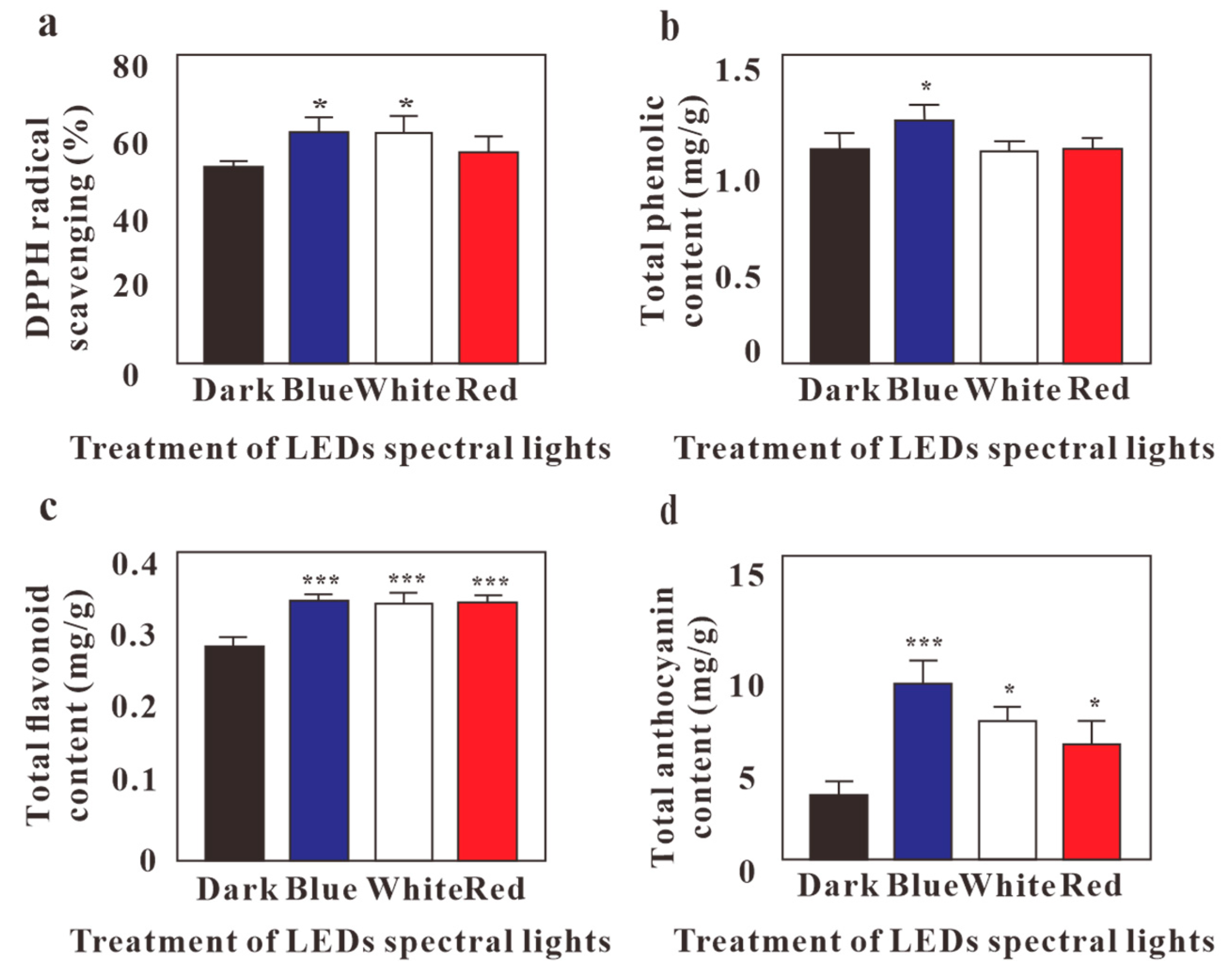
2.2. Effects of LED Lights on Antioxidant Activity, TFC, TPC, and Total Anthocyanin Content of G. Procumbens Calli
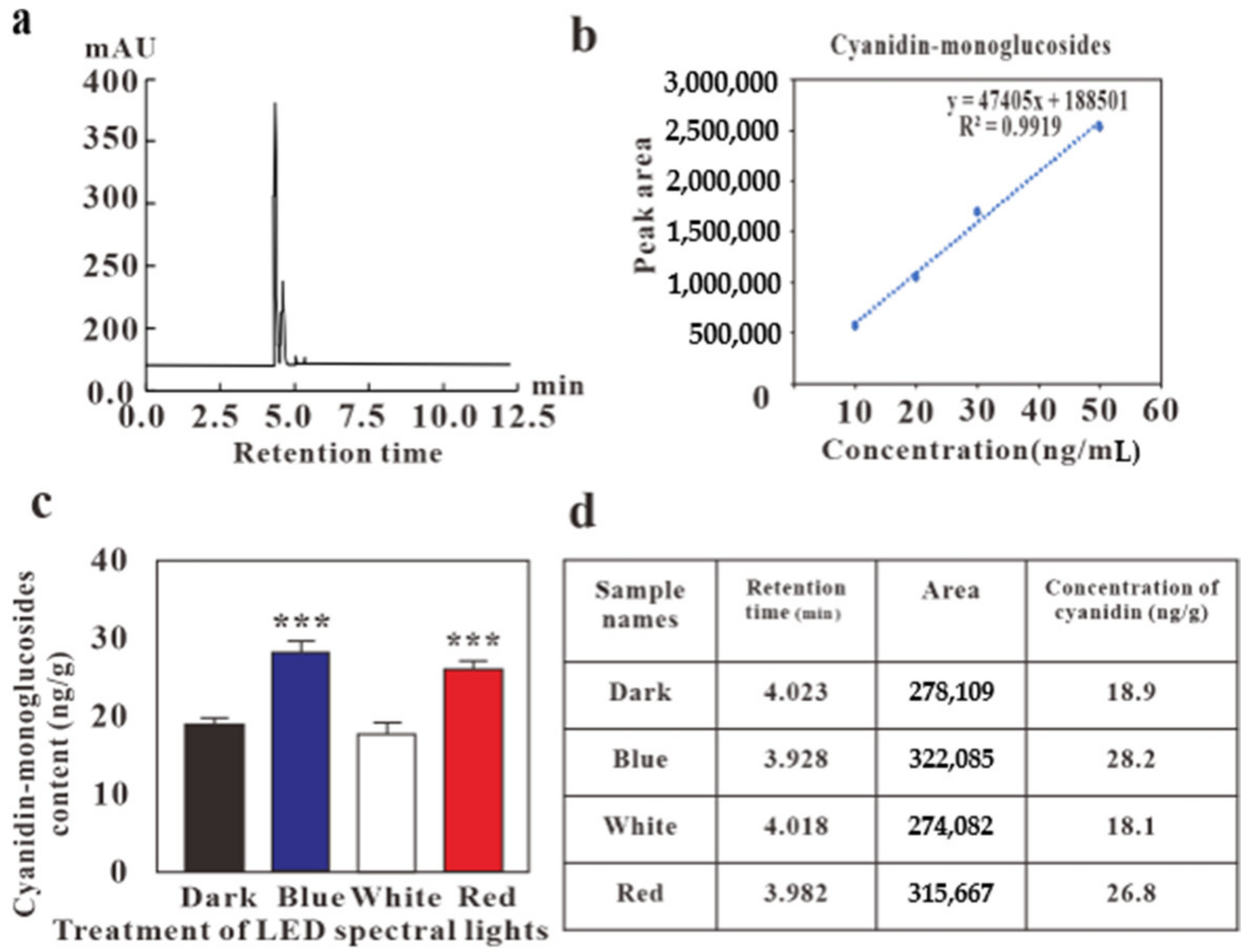
2.3. HPLC and LC-MS Analysis on the Effects of LED Lights on Cyanidin-Monoglucosides Accumulation in Callus
3. Materials and Methods
3.1. Plant Material
3.2. Callus Establishment
3.3. Spectral Light Treatments
3.4. Sample Extraction
3.5. Analysis of Total Phenolic (TPC) and Total Flavonoid (TFC) Contents
3.6. Determination of Antioxidant Activity
3.7. Analysis of Total Anthocyanin Content
3.8. High Performance Liquid Chromatography (HPLC) Analysis
3.9. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franklin, K.A.; Whitelam, G.C. Red: Far-Red ratio perception and shade avoidance. Annu. Plant. Rev. 2007, 211–234. [Google Scholar]
- Kim, S.-J.; Hahn, E.-J.; Heo, J.-W.; Paek, K.-Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Yeh, N.; Chung, J.-P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Kishima, Y.; Shimaya, A.; Adachi, T. Evidence that blue light induces betalain pigmentation in Portulaca callus. Plant. Cell, Tissue Organ. Cult. (PCTOC) 1995, 43, 67–70. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant. Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Tibbitts, T.; Morgan, D.C.; Warrington, I.J. Growth of lettuce, spinach, mustard, and wheat plants under four combinations of high-pressure sodium, metal halide, and tungsten halogen lamps at equal PPFD. J. Am. Soc. Hort. Sci 1983, 108, 622–630. [Google Scholar]
- Alvarenga, I.C.A.; Pacheco, F.V.; Silva, S.T.; Bertolucci, S.K.V.; Pinto, J.E.B.P. In vitro culture of Achillea millefolium L.: Quality and intensity of light on growth and production of volatiles. Plant Cell, Tissue Organ Cult. (PCTOC) 2015, 122, 299–308. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An Overview of LEDs’ Effects on the Production of Bioactive Compounds and Crop Quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Edwards, G.E. Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf coleus varieties. Plant. Cell Physiol. 1996, 37, 395–399. [Google Scholar] [CrossRef]
- Núñez, M.; Magnuson, B.; Wallace, T.; Giusti, M. Anthocyanins in health and disease prevention. Anthocyanins in Health and Dis. 2013, 1–12. [Google Scholar]
- Markakis, P. Anthocyanins as Food Colors Food Science and Technology—A Series of Monographs; Academic Press: New York, NY, USA, 1982; pp. 163–178. [Google Scholar]
- Bondia-Pons, I.; Aura, A.-M.; Vuorela, S.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Rye phenolics in nutrition and health. J. Cereal Sci. 2009, 49, 323–336. [Google Scholar] [CrossRef]
- Han, F.L.; Xu, Y. Effect of the structure of seven anthocyanins on self-association and colour in an aqueous alcohol solution. South. Afr. J. Enol. Vitic. 2015, 36, 105–116. [Google Scholar] [CrossRef]
- Seeram, N. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stoichiometric and kinetic studies of phenolic antioxidants from andean purple corn and red-fleshed sweetpotato. J. Agric. Food Chem. 2003, 51, 3313–3319. [Google Scholar] [CrossRef]
- Chaudhary, B.; Mukhopadhyay, K. Induction of anthocyanin pigment in callus cultures of Solanum melongena L. in response to plant growth regulators and light. IOSR J. Pharm. 2012, 2, 76–80. [Google Scholar]
- Martínez, M.E.; Poirrier, P.; Prüfer, D.; Gronover, C.S.; Jorquera, L.; Ferrer, P.; Díaz, K.; Chamy, R.; Schulze, C. Kinetics and modeling of cell growth for potential anthocyanin induction in cultures of Taraxacum officinale G.H. Weber ex Wiggers (Dandelion) in vitro. Electron. J. Biotechnol. 2018, 36, 15–23. [Google Scholar] [CrossRef]
- Christophe, W. Medicinal Plants of the Asia-Pacific: Drugs for the Future? World Scientific Publishing Co Pte Ltd: Hackensack, NJ, USA, 2006; pp. 434–650. [Google Scholar]
- Puangpronpitag, D.; Chaichanadee, S.; Naowaratwattana, W.; Sittiwet, C.; Thammasarn, K. Evaluation of nutritional value and antioxidative properties of the medicinal plant Gynura procumbens extract. Asian J. Plant. Sci. 2010, 9, 146–151. [Google Scholar]
- Afandi, A.; Sadikun, A.; Ismail, S. Antioxidant properties of Gynura procumbens extracts and their inhibitory effects on two major human recombinant cytochrome P450s using a high throughout luminescence assay. Asian J. Pharm. Clin. Res. 2014, 7, 36–41. [Google Scholar]
- Rosidah; Yam, M.; Sadikun, A.; Asmawi, M. Antioxidant potential of Gynura procumbens. Pharm. Boil. 2008, 46, 616–625. [Google Scholar] [CrossRef]
- Zhang, X.F.; Tan, B.K. Effects of an ethanolic extract of Gynura procumbens on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singap. Med. J. 2000, 41, 9–13. [Google Scholar]
- Akowuah, G.; Sadikun, A.; Mariam, A. Flavonoid identification and hypoglycaemic studies of the butanol fraction from gynura procumbens. Pharm. Boil. 2002, 40, 405–410. [Google Scholar] [CrossRef]
- Dwijayanti, D.R.; Rifa’I, M. Gynura procumbens ethanolic extract promotes lymphocyte activation and regulatory t cell generation in vitro. J. Trop. Life Sci. 2015, 5, 14–19. [Google Scholar]
- Georgiev, V.; Ilieva, M.; Bley, T.; Pavlov, A. Betalain production in plant in vitro systems. Acta Physiol. Plant. 2008, 30, 581–593. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant. Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Arumugam, M.; Pawar, U.R.; Gomathinayagam, M.; Lakshmanan, G.M.A.; Panneerselvam, R. Antibacterial and antioxidant activity between micropropagated and field grown plants of excoecaria agallocha L. 2012. [Google Scholar]
- Zand, R.S.R.; Jenkins, D.J.; Diamandis, E.P. Flavonoids and steroid hormone-dependent cancers. J. Chromatogr. B 2002, 777, 219–232. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Lekkham, P.; Srilaong, V.; Pongprasert, N.; Kondo, S. Anthocyanin concentration and antioxidant activity in light-emitting diode (LED)-treated apples in a greenhouse environmental control system. Fruits 2016, 71, 269–274. [Google Scholar] [CrossRef]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xing, T.; Wang, X. The role of light in the regulation of anthocyanin accumulation in Gerbera hybrida. Plant. Growth Regul. 2004, 44, 243–250. [Google Scholar] [CrossRef]
- Shi, L.; Cao, S.; Chen, W.; Yang, Z. Blue light induced anthocyanin accumulation and expression of associated genes in Chinese bayberry fruit. Sci. Hortic. 2014, 179, 98–102. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009, 114, 1271–1277. [Google Scholar] [CrossRef]
- Lin, C. Blue light receptors and signal transduction. Plant. Cell 2002, 14, S207–S225. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yap, W.S.; Yong, W.T.L. Comparison of anthocyanin and phenolic contents between tuber and callus of ipomoea batatas (L.). Pertanika J. Trop. Agric. Sci. 2012, 35, 9–14. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, T.T.; Moe, M.M.; Kim, Y.J.; Bang, K.S. Effects of Different Colored LEDs on the Enhancement of Biologically Active Ingredients in Callus Cultures of Gynura procumbens (Lour.) Merr. Molecules 2019, 24, 4336. https://doi.org/10.3390/molecules24234336
Lian TT, Moe MM, Kim YJ, Bang KS. Effects of Different Colored LEDs on the Enhancement of Biologically Active Ingredients in Callus Cultures of Gynura procumbens (Lour.) Merr. Molecules. 2019; 24(23):4336. https://doi.org/10.3390/molecules24234336
Chicago/Turabian StyleLian, Thang Tung, Myat Myat Moe, Yong Ju Kim, and Keuk Soo Bang. 2019. "Effects of Different Colored LEDs on the Enhancement of Biologically Active Ingredients in Callus Cultures of Gynura procumbens (Lour.) Merr." Molecules 24, no. 23: 4336. https://doi.org/10.3390/molecules24234336
APA StyleLian, T. T., Moe, M. M., Kim, Y. J., & Bang, K. S. (2019). Effects of Different Colored LEDs on the Enhancement of Biologically Active Ingredients in Callus Cultures of Gynura procumbens (Lour.) Merr. Molecules, 24(23), 4336. https://doi.org/10.3390/molecules24234336





