Grape Berry Flavonoid Responses to High Bunch Temperatures Post Véraison: Effect of Intensity and Duration of Exposure
Abstract
:1. Introduction
2. Results and Discussion
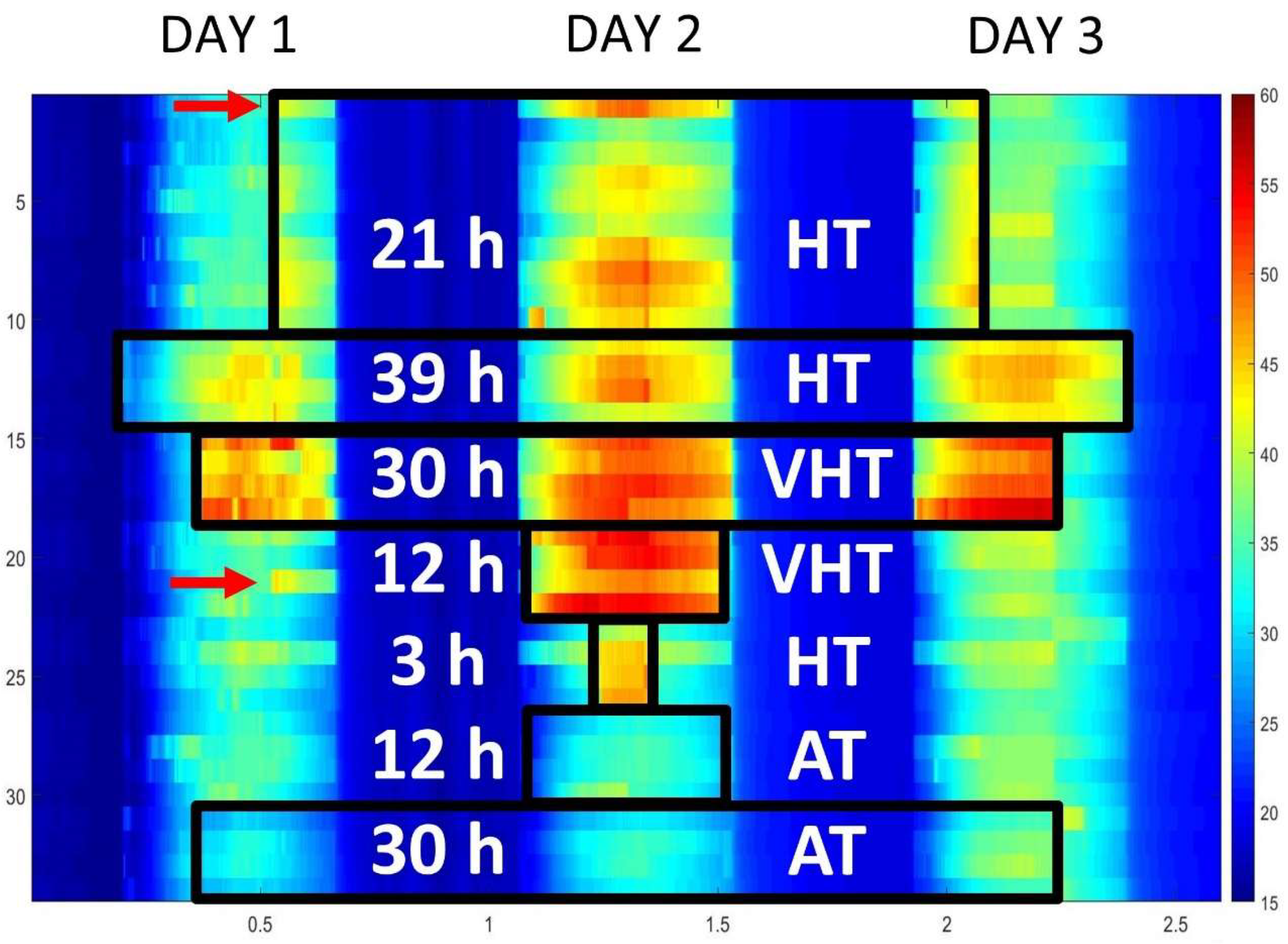
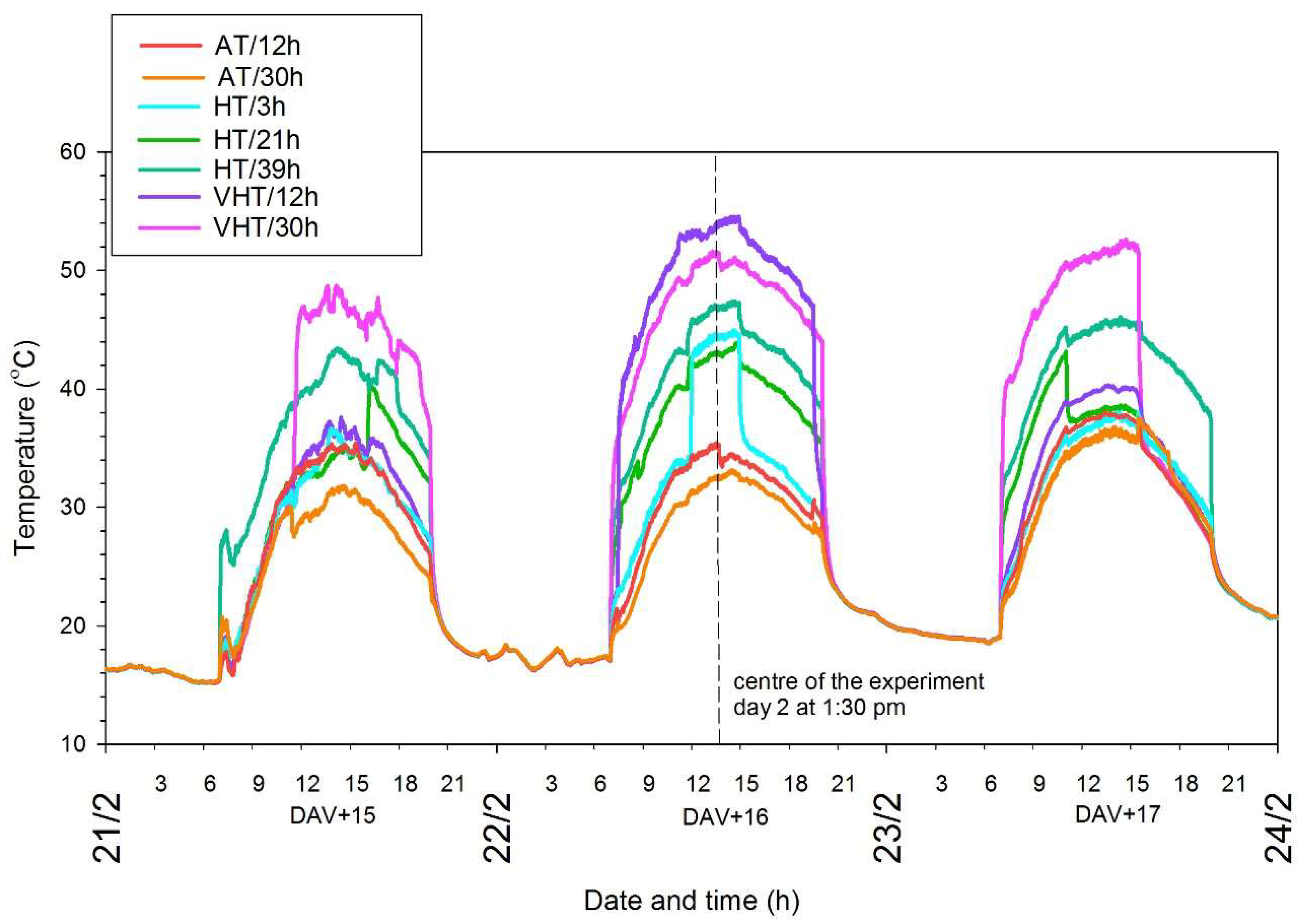
2.1. Experimental Design and Temperature Treatments
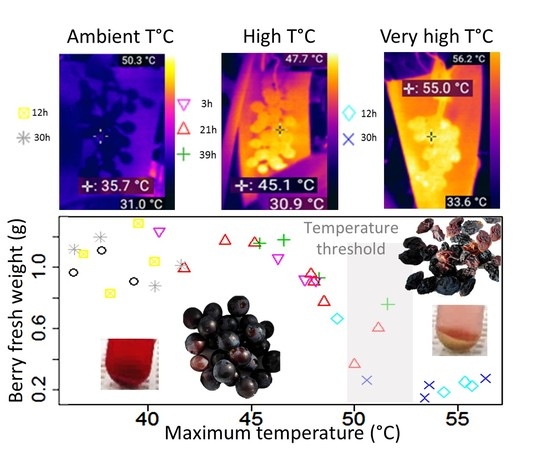
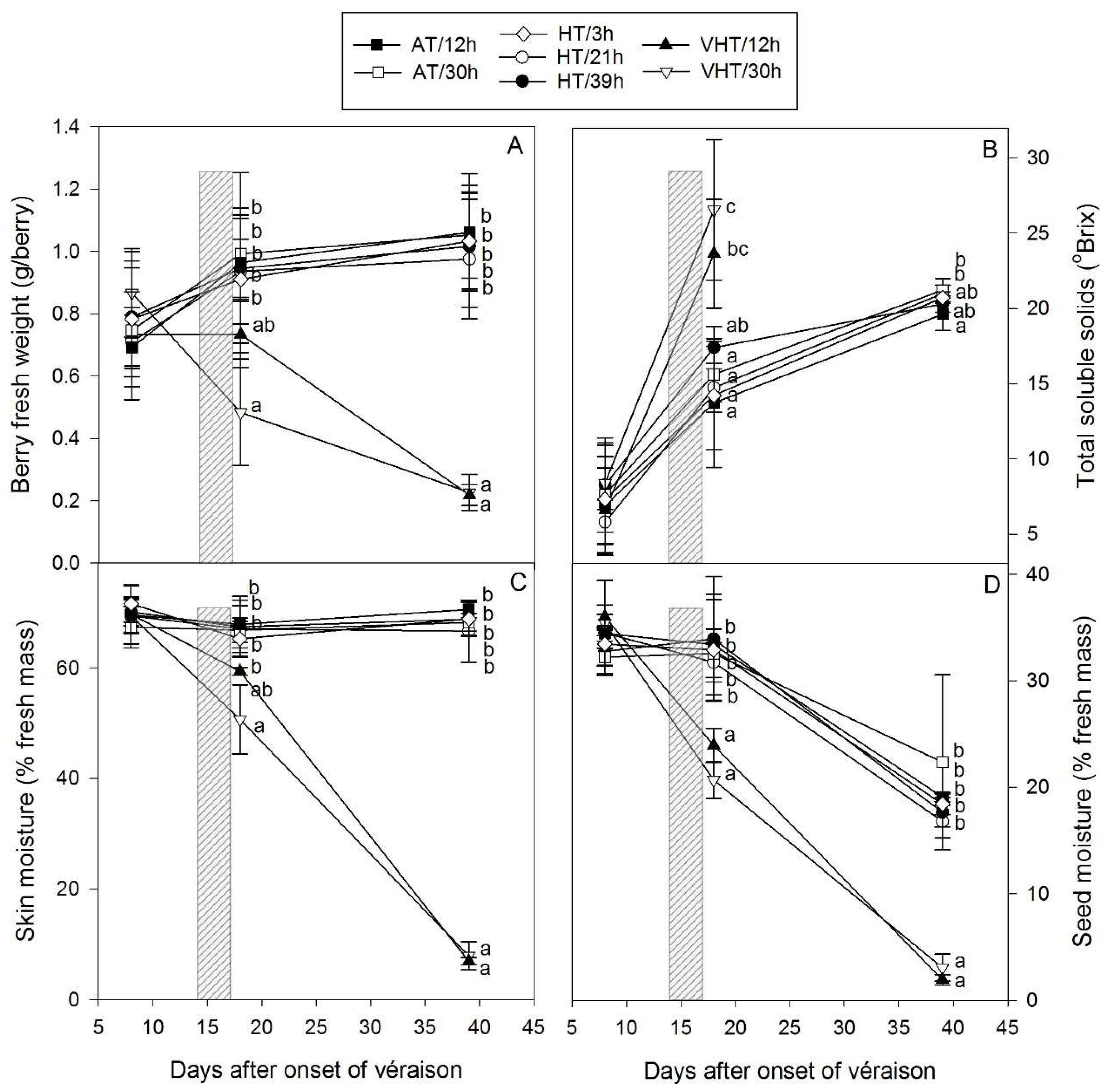
2.2. Berry Physiology Responses to Different Temperature Intensities and Durations
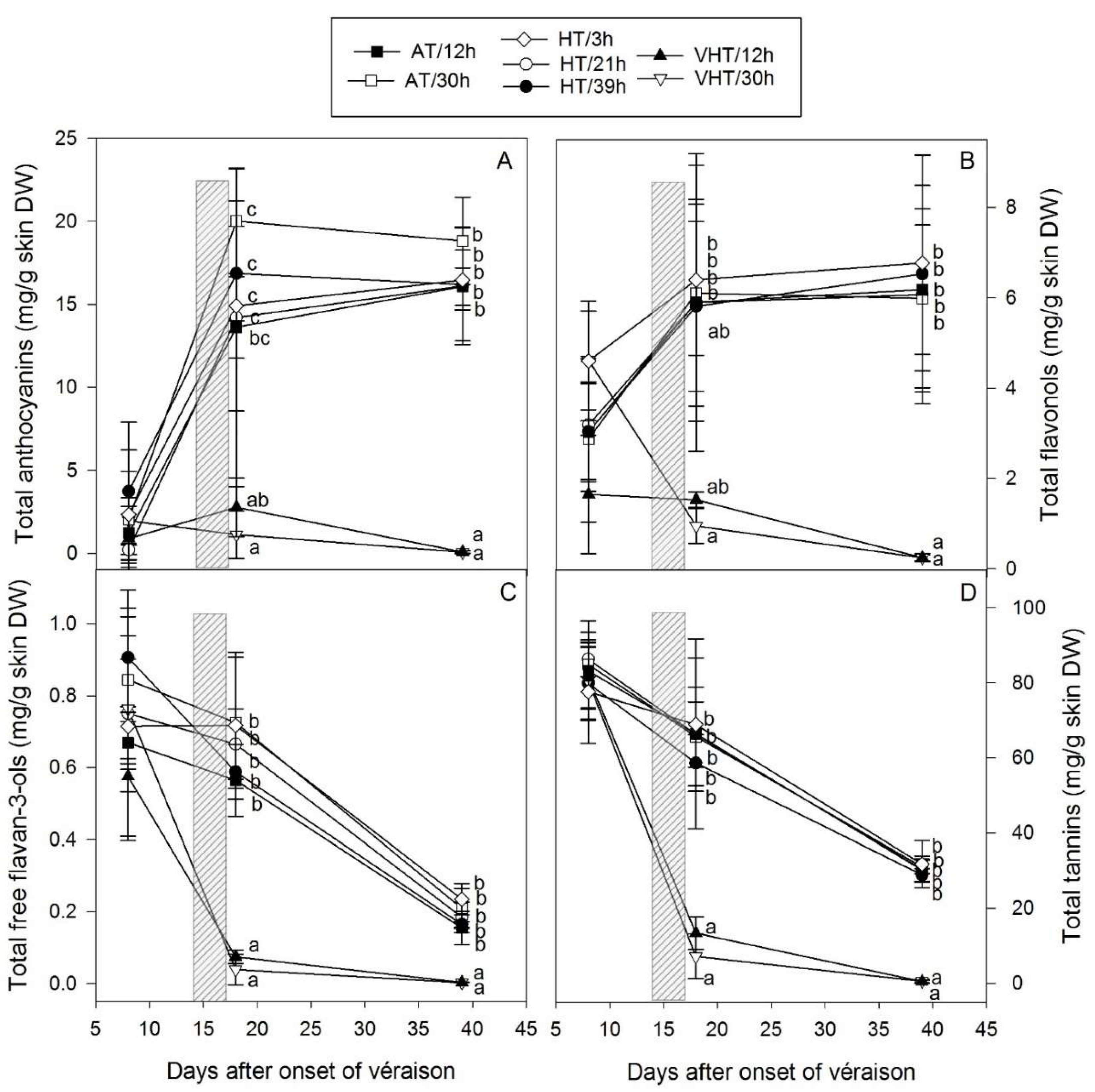
2.3. Temperature Effect on Total Skin Flavonoids
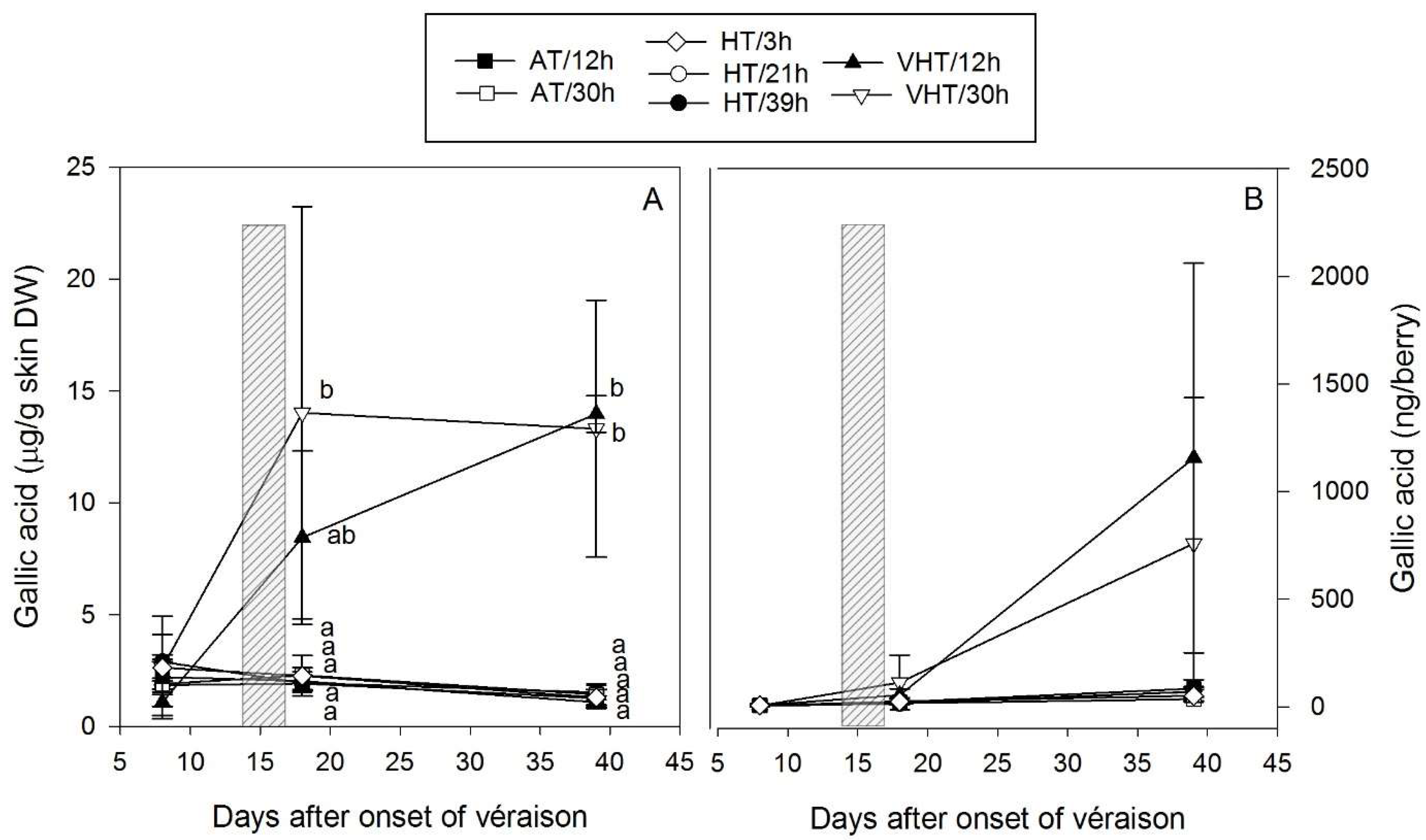
2.4. Temperature Effect on Skin Flavonoid Composition
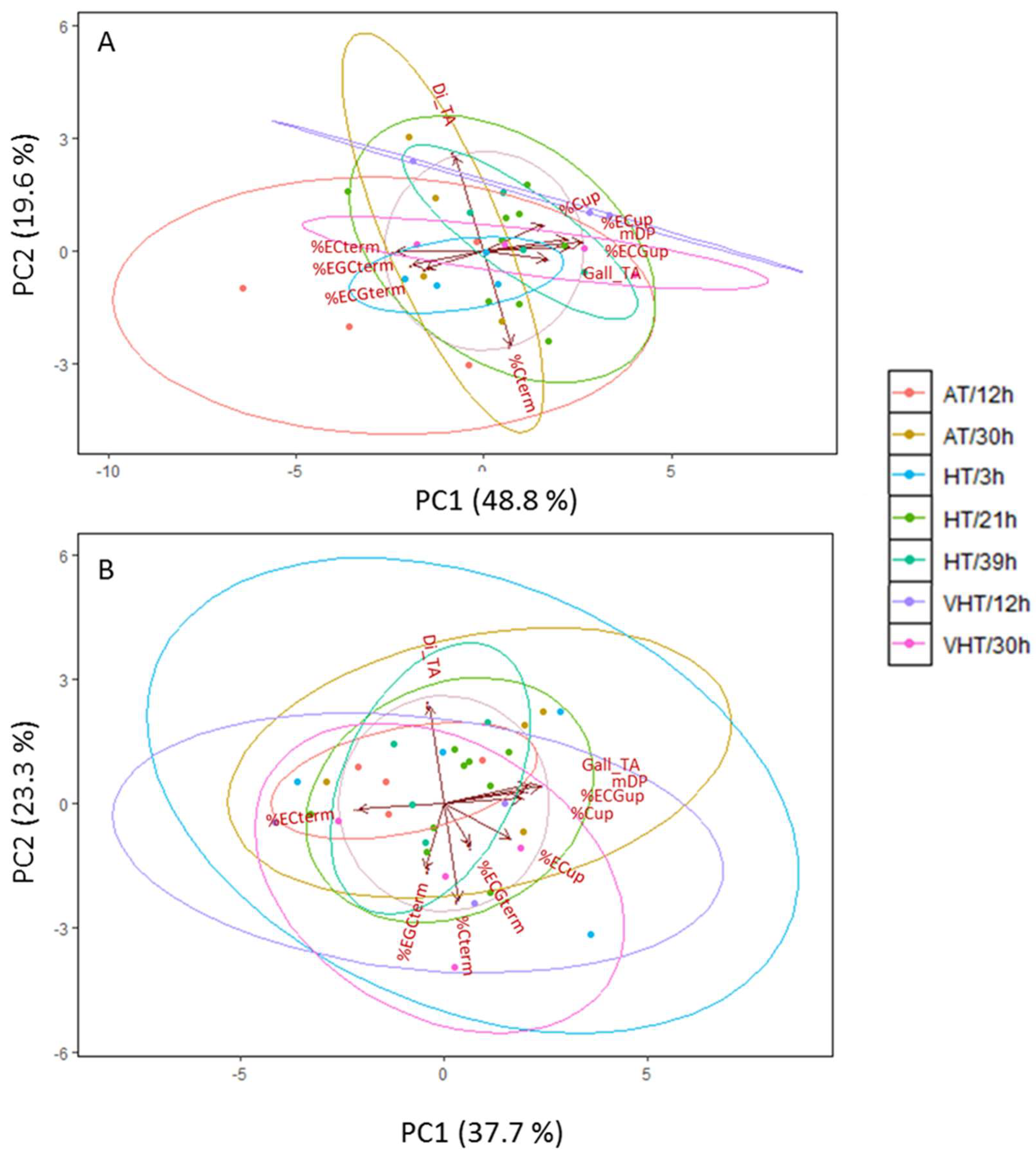
2.5. Temperature Effect on Skin Flavonoid Profile
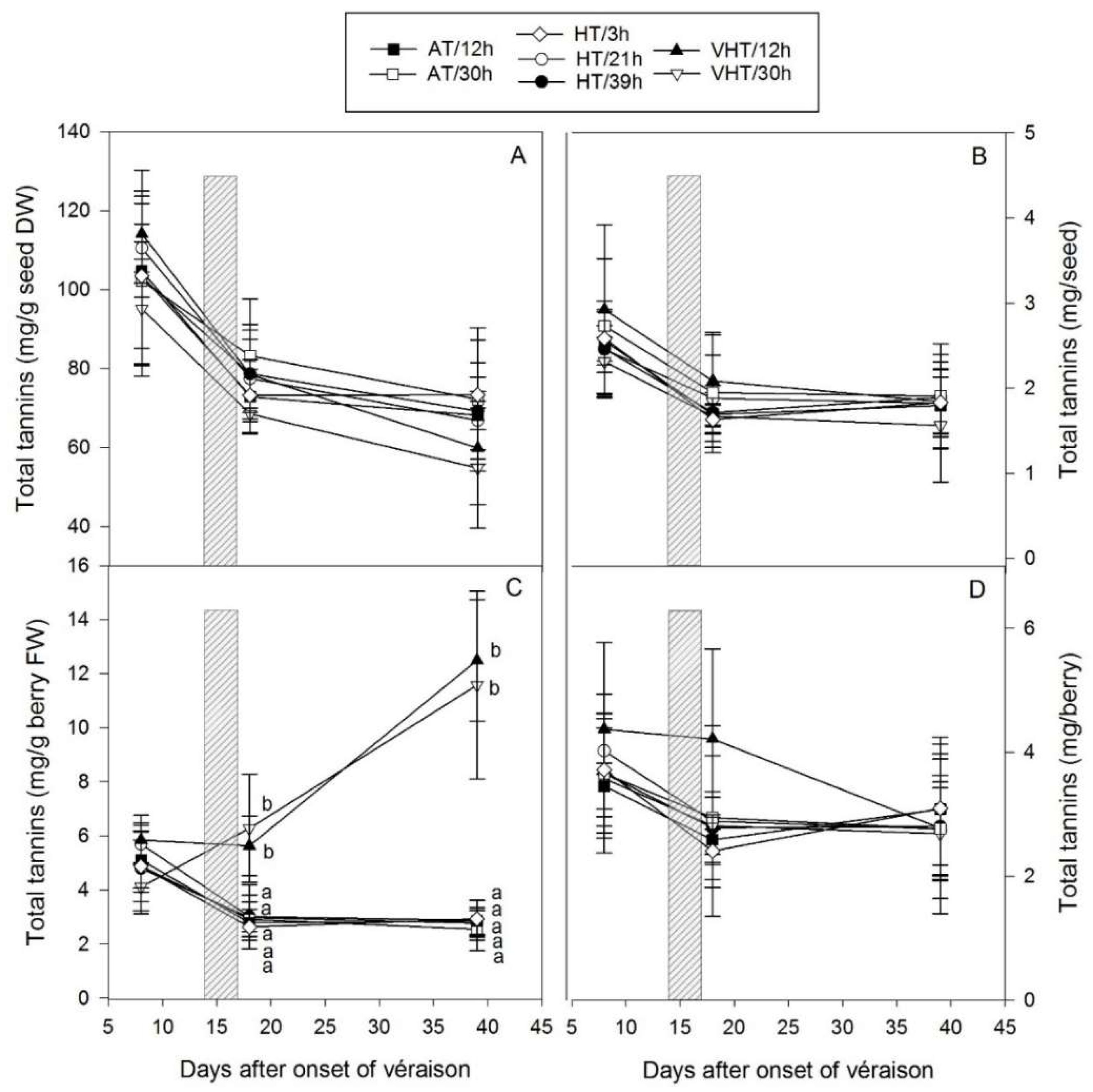
2.6. Temperature Effect on Seed Tannins
3. Materials and Methods
3.1. Plant Materials
3.2. Treatment Application and Design
3.3. Grape Sampling
3.4. Chemical Analysis
3.4.1. Chemical Reagents
3.4.2. Extraction and Analysis
3.5. Chemometrics and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
Appendix C
Appendix D
References
- Perkins, S.E.; Alexander, L.V. On the measurement of heat waves. J. Clim. 2013, 26, 4500–4517. [Google Scholar] [CrossRef]
- Perkins-Kirkpatrick, S.; White, C.; Alexander, L.; Argüeso, D.; Boschat, G.; Cowan, T.; Evans, J.; Ekström, M.; Oliver, E.; Phatak, A. Natural hazards in Australia: Heatwaves. Clim. Chang. 2016, 139, 101–114. [Google Scholar] [CrossRef]
- Perkins-Kirkpatrick, S.; Pitman, A. Extreme events in the context of climate change. Public Health Res. Pract. 2018, 28, e2841825. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.; Whiting, J.; Watt, A.; Hill, T.; Wigg, F.; Dunn, G.; Needs, S.; Barlow, E.W.R. Managing Grapevines through Severe Heat: A Survey of Growers after the 2009 Summer Heatwave in South-eastern Australia. J. Wine Res. 2010, 21, 147–165. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Luciani, E.; Sabbatini, P.; Berrios, J.G.; Palliotti, A. Kaolin treatments on Pinot noir grapevines for the control of heat stress damages. BIO Web Conf. 2019, 13, 04004. [Google Scholar] [CrossRef]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Pieri, P.; Charon, J.; Pillet, J.; Hilbert, G.; Renaud, C.; Gomès, E.; Delrot, S.; Lecourieux, D. Dissecting the biochemical and transcriptomic effects of a locally applied heat treatment on developing Cabernet Sauvignon grape berries. Front. Plant. Sci. 2017, 8, 53. [Google Scholar] [CrossRef]
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between wine composition and temperature: Impact on Bordeaux wine typicity in the context of global warming—Review. Crit. Rev. Food Sci. Nutr. 2017. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Doehlert, D.H. Uniform shell designs. Appl. Stat. 1970, 19, 231–239. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; dos Santos, W.N.L.; Quintella, C.M.; Neto, B.B.; Bosque-Sendra, J.M. Doehlert matrix: A chemometric tool for analytical chemistry—review. Talanta 2004, 63, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Barril, C. Impact of short temperature exposure of Vitis vinifera L. cv. Shiraz grapevine bunches on berry development, primary metabolism and tannin accumulation. Environ. Exp. Bot. 2019, 168, 103866. [Google Scholar] [CrossRef]
- Kliewer, W. Effect of high temperatures during the bloom-set period on fruit-set, ovule fertility, and berry growth of several grape cultivars. Am. J. Enol. Viticult. 1977, 28, 215–222. [Google Scholar]
- Reshef, N.; Fait, A.; Agam, N. Grape berry position affects the diurnal dynamics of its metabolic profile. Plant. Cell Environ. 2019. [Google Scholar] [CrossRef]
- Reshef, N.; Walbaum, N.; Agam, N.; Fait, A. Sunlight Modulates Fruit Metabolic Profile and Shapes the Spatial Pattern of Compound Accumulation within the Grape Cluster. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines: Anatomy and Physiology, 2nd ed.; Academic Press: New York, NY, USA, 2015. [Google Scholar]
- Perkins, S. Scorcher. Available online: http://scorcher.org.au (accessed on 2 February 2019).
- Schrader, L.E.; Zhang, J.; Duplaga, W.K. Two types of sunburn in apple caused by high fruit surface (peel) temperature. Plant. Health Prog. 2001, 2, 3. [Google Scholar] [CrossRef]
- Rousserie, P.; Rabot, A.; Geny-Denis, L. From flavanols biosynthesis to wine tannins: What place for grape seeds? J. Agric. Food Chem. 2019, 67, 1325–1343. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry Temperature and Solar Radiation Alter Acylation, Proportion, and Concentration of Anthocyanin in Merlot Grapes. Am. J. Enol. Viticult. 2008, 59, 235–247. [Google Scholar]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. Pyranoanthocyanins–an overview on structures, occurrence, and pathways of formation. Trends Food Sci. Tech. 2007, 18, 526–534. [Google Scholar] [CrossRef]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Pinasseau, L.; Vallverdú-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Peros, J.-P.; Ageorges, A.; Sommerer, N.; Boulet, J.-C. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Front. Plant. Sci. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Arapitsas, P.; Oliveira, J.; Mattivi, F. Do white grapes really exist? Food Res. Int. 2015, 69, 21–25. [Google Scholar] [CrossRef]
- Zhao, Q.; Duan, C.-Q.; Wang, J. Anthocyanins profile of grape berries of Vitis amurensis, its hybrids and their wines. Int. J. Mol. Sci. 2010, 11, 2212–2228. [Google Scholar] [CrossRef] [PubMed]
- Degu, A.; Ayenew, B.; Cramer, G.R.; Fait, A. Polyphenolic responses of grapevine berries to light, temperature, oxidative stress, abscisic acid and jasmonic acid show specific developmental-dependent degrees of metabolic resilience to perturbation. Food Chem. 2016, 212, 828–836. [Google Scholar] [CrossRef]
- Rustioni, L. Oxidized polymeric phenolics: Could they be considered photoprotectors? J. Agric. Food Chem. 2017, 65, 7843–7846. [Google Scholar] [CrossRef]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Viticult. 2002, 53, 171–182. [Google Scholar]
- Downey, M.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Viticult. 2006, 57, 257–268. [Google Scholar]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 2003, 9, 110–121. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature on grape phenolic metabolism. Anal. Chim. Acta. 2008, 621, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Pastore, C.; Dal Santo, S.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole plant temperature manipulation affects flavonoid metabolism and the transcriptome of grapevine berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low night temperature at veraison enhances the accumulation of anthocyanins in Corvina grapes (Vitis Vinifera L.). Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gao-Takai, M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. A low temperature promotes anthocyanin biosynthesis but does not accelerate endogenous abscisic acid accumulation in red-skinned grapes. Plant. Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; D’Incà, E.; Tornielli, G.B.; Filippetti, I. The grapevine VviPrx31 peroxidase as a candidate gene involved in anthocyanin degradation in ripening berries under high temperature. J. Plant. Res. 2016, 129, 513–526. [Google Scholar] [CrossRef]
- Saeidian, S.; Ghasemifar, E. Effect of Temperature on Guaiacol Peroxidase of Pyrus communis. Int. Lett. Nat. Sci. 2013, 5, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Chamorro, S.; Goñi, I.; Viveros, A.; Hervert-Hernández, D.; Brenes, A. Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur. Food Res.Technol. 2012, 234, 147–155. [Google Scholar] [CrossRef]
- Ross, C.F.; Hoye, C., Jr.; Fernandez-Plotka, V.C. Influence of heating on the polyphenolic content and antioxidant activity of grape seed flour. J. Food Sci. 2011, 76, C884–C890. [Google Scholar] [CrossRef] [PubMed]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.-M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant. Biol. 2016, 16, 164. [Google Scholar] [CrossRef] [PubMed]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant. Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Brillouet, J.-M.; Fulcrand, H.; Carrillo, S.; Rouméas, L.; Romieu, C. Isolation of Native Proanthocyanidins from Grapevine (Vitis vinifera) and Other Fruits in Aqueous Buffer. J. Agric. Food Chem. 2017, 65, 2895–2901. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Effect of high temperature on anthocyanin composition and transcription of flavonoid hydroxylase genes in ‘Pinot noir’grapes (Vitis vinifera). J. Hortic. Sci. Bio. Tech. 2007, 82, 199–206. [Google Scholar] [CrossRef]
- Sommer, K.; Edwards, E.; Unwin, D.; Mazza, M.; Downey, M. Strategies to Maintain Productivity and Quality in a Changing Environment-Impacts of Global Warming on Grape and Wine Production; Future Farming Systems Research: Victoria, Australia, 2012. [Google Scholar]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Barril, C. Single and cumulative effects of whole-vine heat events on Shiraz berry composition. OENO One 2019, 53. [Google Scholar] [CrossRef]
- Downey, M.; Harvey, J.S.; Robinson, S.P. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2004, 10, 55–73. [Google Scholar] [CrossRef]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- García-Marino, M.; Rivas-Gonzalo, J.C.; Ibáñez, E.; García-Moreno, C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal. Chim. Acta. 2006, 563, 44–50. [Google Scholar] [CrossRef]
- Romanini, E.; McRae, J.M.; Colangelo, D.; Lambri, M. First trials to assess the feasibility of grape seed powder (GSP) as a novel and sustainable bentonite alternative. Food Chem. 2019, 125484. [Google Scholar] [CrossRef] [PubMed]
- Shrikhande, A.J. Wine by-products with health benefits. Food Res. Int. 2000, 33, 469–474. [Google Scholar] [CrossRef]
- Coombe, B. Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Gouthu, S.; O’Neil, S.T.; Di, Y.; Ansarolia, M.; Megraw, M.; Deluc, L.G. A comparative study of ripening among berries of the grape cluster reveals an altered transcriptional programme and enhanced ripening rate in delayed berries. J. Exp. Bot. 2014, 65, 5889–5902. [Google Scholar] [CrossRef]
- Pinasseau, L.; Verbaere, A.; Roques, M.; Meudec, E.; Vallverdú-Queralt, A.; Terrier, N.; Boulet, J.-C.; Cheynier, V.; Sommerer, N. A fast and robust UHPLC-MRM-MS method to characterize and quantify grape skin tannins after chemical depolymerization. Molecules 2016, 21, 1409. [Google Scholar] [CrossRef] [Green Version]
- Sarneckis, C.J.; Dambergs, R.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Treatments | Coded Values | Targeted Values 1 | Experimental T Values | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X1 (h) | X2 (°C) | dT (°C) | Tmean (°C) | Tmax (°C) | |
| AT/12h | −0.5 | −0.866 | 12 | 0.01 | +0.05 | 31.2 | 35.9 |
| AT/30h | 0.5 | −0.866 | 30 | 0.01 | −1.8 | 31.6 | 33.3 |
| HT/3h | −1 | 0 | 3 | +8.36 | +10.2 | 43.8 | 45.7 |
| HT/21h | 0 | 0 | 21 | +8.36 | +7.3 | 37.6 | 45.1 |
| HT/39h | 1 | 0 | 39 | +8.36 | +9.0 | 39.7 | 46.7 |
| VHT/12h | −0.5 | 0.866 | 12 | +16.71 | +19.8 | 49.9 | 55.1 |
| VHT/30h | 0.5 | 0.866 | 30 | +16.71 | +14.6 | 46.6 | 52.2 |
| Coefficients | Anthocyanins | Flavonols | Flavan-3-ols | Tannins | |||||
|---|---|---|---|---|---|---|---|---|---|
| DAV+18 | DAV+39 | DAV+18 | DAV+39 | DAV+18 | DAV+39 | DAV+18 | DAV+ 39 | ||
| Constant | b0 | 12.896 *** | 12.828 *** | 5.313 *** | 5.436 *** | 0.604 *** | 0.171 *** | 82.069 *** | 37.807 *** |
| Duration | b1 | 0.724 | −0.782 | 0.512 | 0.320 | 0.0144 | −0.0129 | −2.495 | −0.601 |
| dT | b2 | −7.070 *** | −8.373 *** | −2.070 * | −2.667 ** | −0.3 *** | 0.104 *** | −35.867 *** | −21.593 *** |
| Duration * dT | b12 | −5.233 * | −2.366 | −1.685 | −0.822 | −0.0733 | −0.0207 | −4.603 | −0.472 |
| Regression | R2 | 0.426 | 0.597 | 0.193 | 0.246 | 0.401 | 0.531 | 0.389 | 0.568 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Barril, C. Grape Berry Flavonoid Responses to High Bunch Temperatures Post Véraison: Effect of Intensity and Duration of Exposure. Molecules 2019, 24, 4341. https://doi.org/10.3390/molecules24234341
Gouot JC, Smith JP, Holzapfel BP, Barril C. Grape Berry Flavonoid Responses to High Bunch Temperatures Post Véraison: Effect of Intensity and Duration of Exposure. Molecules. 2019; 24(23):4341. https://doi.org/10.3390/molecules24234341
Chicago/Turabian StyleGouot, Julia C., Jason P. Smith, Bruno P. Holzapfel, and Celia Barril. 2019. "Grape Berry Flavonoid Responses to High Bunch Temperatures Post Véraison: Effect of Intensity and Duration of Exposure" Molecules 24, no. 23: 4341. https://doi.org/10.3390/molecules24234341