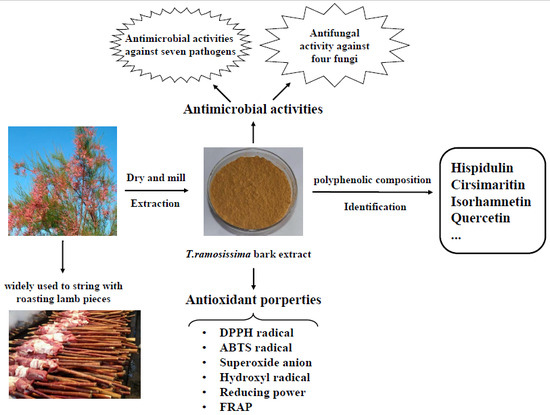
Isorhamnetin, Hispidulin, and Cirsimaritin Identified in Tamarix ramosissima Barks from Southern Xinjiang and Their Antioxidant and Antimicrobial Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Content and Variety of Total Polyphenolics from T. ramosissima Barks
2.1.1. TPC and Total Flavonoid Content (TFC)
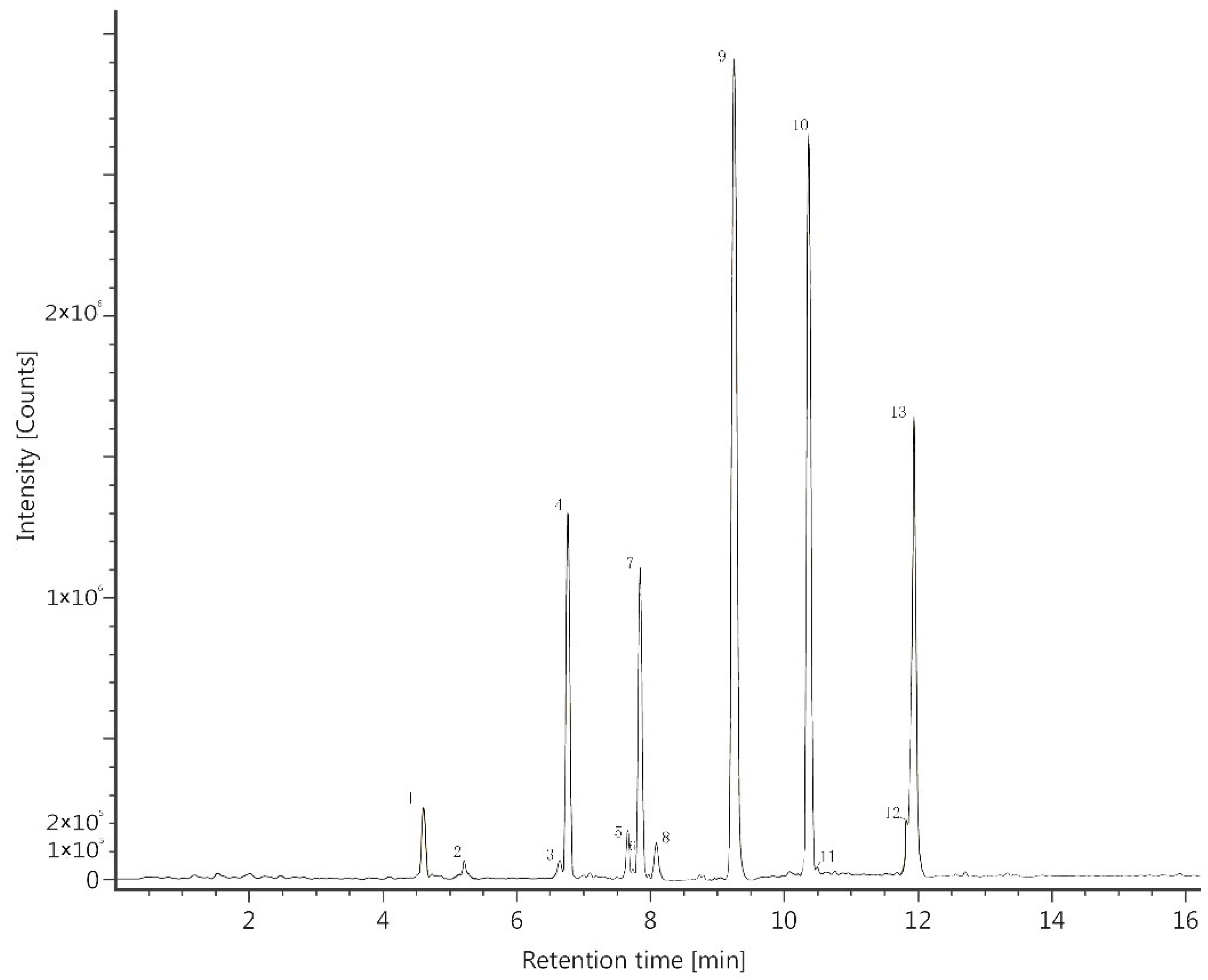
2.1.2. Variety and Content of the Polyphenolics
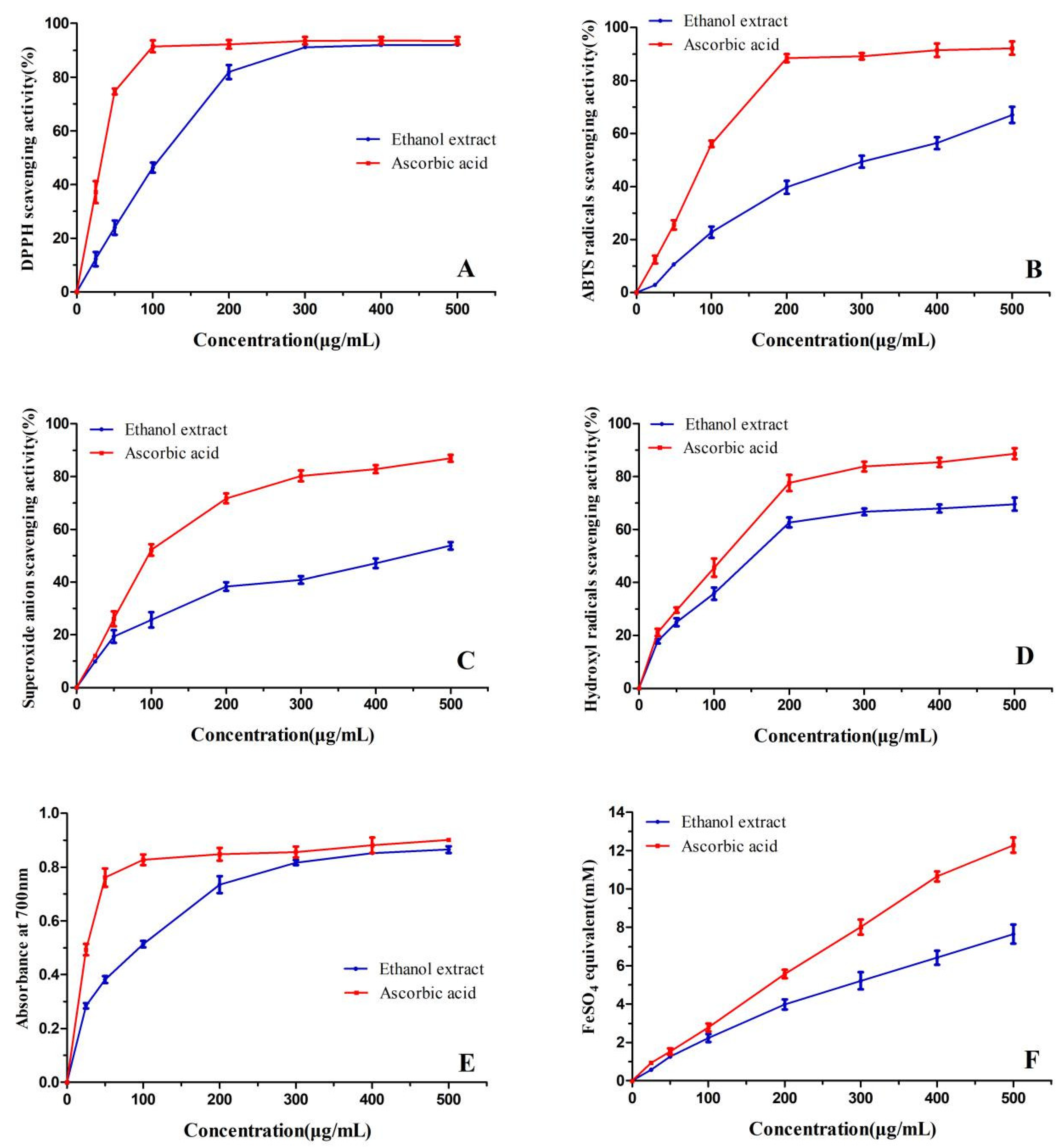
2.2. Antioxidant Activity of the Bark Extract
2.2.1. DPPH Scavenging Activity
2.2.2. 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) Scavenging Activity
2.2.3. Superoxide Anion Scavenging Activity
2.2.4. Hydroxyl Radicals Scavenging Activity
2.2.5. Reducing Power
2.2.6. Ferric Reducing Antioxidant Power (FRAP)
2.3. Antimicrobial Activity
3. Materials and Methods
3.1. Plant Materials and Extraction Procedure
3.2. Determination of Total Polyphenolic Content
3.3. Determination of Total Flavonoid Content
3.4. Analysis of Bark Extract by UPLC-MS
3.5. Determination of Antioxidant Assays
3.5.1. DPPH Radical Scavenging Activity
3.5.2. ABTS Radical Scavenging Activity
3.5.3. Superoxide Anion Radical Scavenging Activity
3.5.4. Hydroxyl Radical Scavenging Activity
3.5.5. Reducing Power
3.5.6. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. Determination of Antimicrobial Activity
3.6.1. Microorganisms
3.6.2. Agar Disc Diffusion Method
3.6.3. Determination of Minimum Inhibitory Concentration (MIC)
3.6.4. Determination of Minimum Bactericidal Concentration (MBC)
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sultanova, N.; Makhmoor, T.; Abilov, Z.A.; Parween, Z.; Omurkamzinova, V.B.; Atta ur, R.; Choudhary, M.I. Antioxidant and antimicrobial activities of Tamarix ramosissima. J. Ethnopharmacol. 2001, 78, 201–205. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sultana, S. Tamarix gallica ameliorates thioacetamide-induced hepatic oxidative stress and hyperproliferative response in Wistar rats. J. Enzym. Inhib. Med. Chem. 2006, 21, 215–223. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, C.S.; Sun, N.; Li, W.Q.; Niu, Y.; Han, H.Q.; Miao, Z.H.; Zhao, X.X.; Zhao, J.; Li, J. Tamaractam, a new bioactive Lactam from Tamarix ramosissima, induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes. Molecules 2017, 22, 96. [Google Scholar] [CrossRef]
- Rahman, M.A.; Haque, E.; Hasanuzzaman, M.; Shahid, I.Z. Antinociceptive, Antiinflammatory and antibacterial properties of Tamarix indica roots. Int. J. Pharmacol. 2011, 7, 527–531. [Google Scholar] [CrossRef]
- Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Mhamdi, B.; Chaieb, K.; Bakrouf, A.; Magne, C.; Abdelly, C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 2009, 47, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Shibamoto, T.; Takeoka, G.R.; Lee, S.E.; Kim, J.H.; Park, B.S. Inhibitory effects of plant-derived flavonoids and phenolic acids on malonaldehyde formation from ethyl arachidonate. J. Agric. Food Chem. 2003, 51, 7203–7207. [Google Scholar] [CrossRef]
- Abouzid, S.F.; Ali, S.A.; Choudhary, M.I. A new ferulic acid ester and other constituents from Tamarix nilotica leaves. Chem. Pharm. Bull. 2009, 57, 740–742. [Google Scholar] [CrossRef]
- Bikbulatova, T.N.; Korul’kina, L.M. Composition of Tamarix hokenakeri and T. ramosissima. Chem. Nat. Compd. 2001, 37, 216–218. [Google Scholar] [CrossRef]
- Mohammedi, Z.; Atik, F. Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) Karst. Int. J. Pharm. Biol. Sci. 2011, 2, 609–615. [Google Scholar]
- Parmar, V.S.; Bisht, K.S.; Sharma, S.K.; Jain, R.; Taneja, P.; Singh, S.; Simonsen, O.; Boll, P.M. Highly oxygenated bioactive flavones from Tamarix. Phytochemistry 1994, 36, 507–511. [Google Scholar] [CrossRef]
- Umbetova, A.K.; Choudhary, M.I.; Sultanova, N.A.; Burasheva, G.S.; Abilov, Z.A. Flavonoids of plants from the genus Tamarix. Chem. Nat. Compd. 2005, 41, 728–729. [Google Scholar] [CrossRef]
- Gao, H.; Wang, H.; Peng, J. Hispidulin induces apoptosis through mitochondrial dysfunction and inhibition of P13k/Akt signalling pathway in HepG2 cancer cells. Cell Biochem. Biophys. 2014, 69, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, H.; Ma, L.; Ma, X.; Yin, J.; Wu, S.; Huang, H.; Li, Y. Cirsimaritin inhibits influenza A virus replication by downregulating the NF-κB signal transduction pathway. Virol. J. 2018, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Sultanova, N.; Makhmoor, T.; Yasin, A.; Abilov, Z.A.; Omurkamzinova, V.B.; Atta ur, R.; Choudhary, M.I. Isotamarixen—A new antioxidant and prolyl endopeptidase-inhibiting triterpenoid from Tamarix hispida. Planta Med. 2004, 70, 65–67. [Google Scholar] [CrossRef]
- Ishak, M.S.; El-Sissi, H.I.; Nawwar, M.A.; El-Sherbieny, A.E. Tannins and polyphenolics of the galls of Tamarix aphylla I. Planta Med. 1972, 21, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Wojdylo, A.; Lamer-Zarawska, E.; Swiader, K. Antioxidant tannins from Rosaceae plant roots. Food Chem. 2007, 100, 579–583. [Google Scholar] [CrossRef]
- Yagi, K. A rapid method for evaluation of autoxidation and antioxidants. Agric. Biol. Chem. 1970, 34, 142–145. [Google Scholar] [CrossRef]
- Husain, S.R.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E. Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal. Food Chem. 2005, 92, 193–202. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.W.; Mat-Junit, S.; Aminudin, N.; Ismail, A.; Abdul-Aziz, A. Antioxidant activities and polyphenolics from the shoots of Barringtonia racemosa (L.) Spreng in a polar to apolar medium system. Food Chem. 2012, 134, 324–332. [Google Scholar] [CrossRef]
- Liu, L.; Sun, Y.; Laura, T.; Liang, X.; Ye, H.; Zeng, X. Determination of polyphenolic content and antioxidant activity of kudingcha made from Ilex kudingcha C.J. Tseng. Food Chem. 2009, 112, 35–41. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sanchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Seo, S.; Seo, K.; Ki, S.H.; Shin, S.M. Isorhamnetin inhibits reactive oxygen species-dependent hypoxia inducible factor (HIF)-1α accumulation. Biol. Pharm. Bull. 2016, 39, 1830–1838. [Google Scholar] [CrossRef]
- Quan, Z.; Gu, J.; Dong, P.; Lu, J.; Wu, X.; Wu, W.; Fei, X.; Li, S.; Wang, Y.; Wang, J.; et al. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to cirsimaritin-induced apoptosis in human gallbladder carcinoma GBC-SD cells. Cancer Lett. 2010, 295, 252–259. [Google Scholar] [CrossRef]
- Kim, B.H.; Choi, J.S.; Yi, E.H.; Lee, J.K.; Won, C.; Ye, S.K.; Kim, M.H. Relative antioxidant activities of quercetin and its structurally related substances and their effects on NF-κB/CRE/AP-1 signaling in murine macrophages. Mol. Cells 2013, 35, 410–420. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Wu, Y.; Lin, L.; Wang, J.; Wu, Y.; Chen, Y.; Yi, Z.; Liu, M.; Pang, X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011, 102, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Zheng, R.L.; Jia, Z.J.; Ju, Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef]
- Osman, W.J.A.; Mothana, R.A.; Basudan, O.; Mohammed, M.S.; Mohamed, M.S. Antibacterial effect and radical scavenging activity of hispidulin and nepetin; a two flvaones from Tarconanthus camphoratus L. World J. Pharm. Res. 2015, 4, 424–433. [Google Scholar]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Esteban-Fernandez, A.; Zorraquin-Pena, I.; Ferrer, M.D.; Mira, A.; Bartolome, B.; Gonzalez de Llano, D.; Victoria Moreno-Arribas, M. Inhibition of oral pathogens adhesion to human gingival fibroblasts by wine polyphenols alone and in combination with an oral probiotic. J. Agric. Food Chem. 2018, 66, 2071–2082. [Google Scholar] [CrossRef]
- Yang, L.C.; Li, R.; Tan, J.; Jiang, Z.T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef]
- Liu, F.; Ooi, V.E.C.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.J. Evaluation of free hydroxyl radical scavenging activities of some Chinese herbs by capillary zone electrophoresis with amperometric detection. Anal. Bioanal. Chem. 2004, 378, 1801–1805. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, W.; Yuan, Q.; Ye, H.; Sun, Y.; Zhang, H.; Zeng, X. Box-Behnken design for extraction optimization, characterization and in vitro antioxidant activity of Cicer arietinum L. hull polysaccharides. Carbohydr. Polym. 2016, 147, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Giovanelli, S.; Rocchigiani, G.; Pistelli, L.; Mancianti, F. Antibacterial and antifungal activity of essential oils against some pathogenic bacteria and yeasts shed from poultry. Flavour Frag. J. 2016, 31, 302–309. [Google Scholar] [CrossRef]
- Bassanetti, I.; Carcelli, M.; Buschini, A.; Montalbano, S.; Leonardi, G.; Pelagatti, P.; Tosi, G.; Massi, P.; Fiorentini, L.; Rogolino, D. Investigation of antibacterial activity of new classes of essential oils derivatives. Food Control 2017, 73, 606–612. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (isorhamnetin, hispidulin, cirsimaritin, and quercetin) are not available from the authors. |
| Tamarix Species | Tested Part | Location | TPC (mg GAE/g) | TFC (mg QE/g) | Reference |
|---|---|---|---|---|---|
| T. ramosissima | barks | South Xinjiang, China | 323.45 ± 21.41 | 87.32 ± 1.65 | This work |
| T. gallica | leaves | South Tunis | 34.44 ± 3.40 | 3.91 ± 0.45 | Ksouri et al. [5] |
| T. gallica | flowers | South Tunis | 135.35 ± 7.70 | 12.33 ± 2.10 | Ksouri et al. [5] |
| T. aphylla | leaves | South Algeria | 199.54 ± 1.60 | ND | Mohammedi [9] |
| Peak No. | Polyphenolic Compounds | Retention Time (min) | Empirical Formula | Calcd m/z | Obsd m/z [M + H]+ | Obsd m/z [M − H]+ |
|---|---|---|---|---|---|---|
| 1 | Quercetin 3-O-glucuronide | 4.63 | C21H18O13 | 478.0747 | 479.0820 | 477.0676 |
| 2 | Kaempferol 3-O-glucuronide | 5.19 | C21H18O12 | 462.0798 | 463.0882 | 461.0802 |
| 3 | Eriodictyol | 6.63 | C15H12O6 | 288.0634 | 289.0703 | 287.0581 |
| 4 | Quercetin | 6.77 | C15H10O7 | 302.0427 | 303.0498 | 301.0404 |
| 5 | Naringenin | 7.65 | C15H12O5 | 272.0685 | 273.0753 | 271.0583 |
| 6 | Tangeretin | 7.72 | C20H20O7 | 372.1209 | 373.1274 | 371.1253 |
| 7 | Kaempferol | 7.85 | C15H10O6 | 286.0477 | 287.0547 | 285.0405 |
| 8 | Hesperetin | 8.07 | C16H14O6 | 302.0790 | 303.0856 | 301.0718 |
| 9 | Isorhamnetin | 9.25 | C16H12O7 | 316.0583 | 317.0653 | 315.0510 |
| 10 | Hispidulin | 10.41 | C16H12O6 | 300.0634 | 301.0705 | 299.0562 |
| 11 | Apigenin | 10.42 | C15H10O5 | 270.0528 | 271.0597 | 269.0427 |
| 12 | Glycitein | 11.90 | C16H12O5 | 284.0685 | 285.0754 | 283.0664 |
| 13 | Cirsimaritin | 11.91 | C17H14O6 | 314.0790 | 315.0863 | 313.0717 |
| Compounds | Concentration (μg/mg) |
|---|---|
| Isorhamnetin | 36.9055 |
| Hispidulin | 28.7915 |
| Cirsimaritin | 13.3513 |
| Quercetin | 4.2065 |
| Bacterial Strains | The Extract Concentrations (mg/mL) | Diameter of Inhibition Zones | MIC (mg/mL) | MBC (mg/mL) | ||
|---|---|---|---|---|---|---|
| Bark Extract | Gentamycin (10 UI) | |||||
| Gram- positive strains | Staphylococcus aureus | 1 | 10.60 ± 0.54 A | 20.90 ± 0.14 | 5 | 15 |
| 5 | 11.08 ± 0.71 A | |||||
| 10 | 11.16 ± 0.91 B | |||||
| Listeria monocytogenes | 1 | 10.14 ± 0.48 cAB | 20.30 ± 0.42 | 5 | 10 | |
| 5 | 11.18 ± 0.21 bA | |||||
| 10 | 12.26 ± 0.61 aA | |||||
| Bacillus cereus | 1 | 9.53 ± 0.62 bBC | 18.00 ± 0.00 | 5 | 20 | |
| 5 | 9.72 ± 0.16 bB | |||||
| 10 | 10.88 ± 0.30 aB | |||||
| Gram- negative strains | Escherichia coli | 1 | 9.26 ± 0.65 bC | 17.80 ± 0.28 | 10 | 25 |
| 5 | 9.58 ± 0.34 bB | |||||
| 10 | 10.68 ± 0.81 aB | |||||
| Pseudomonas aeruginosa | 1 | 8.20 ± 0.51 bD | 17.10 ± 0.14 | >10 | NA | |
| 5 | 8.85 ± 0.70 abC | |||||
| 10 | 9.38 ± 0.28 aC | |||||
| Salmonella typhimurium | 1 | 8.00 ± 0.24 bD | 21.75 ± 0.35 | >10 | NA | |
| 5 | 8.34 ± 0.66 bC | |||||
| 10 | 9.48 ± 0.52 aC | |||||
| Shigella castellani | 1 | 10.65 ± 0.45 A | 20.90 ± 0.14 | 5 | 15 | |
| 5 | 10.74 ± 0.50 A | |||||
| 10 | 11.16 ± 0.69 B | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, Hispidulin, and Cirsimaritin Identified in Tamarix ramosissima Barks from Southern Xinjiang and Their Antioxidant and Antimicrobial Activities. Molecules 2019, 24, 390. https://doi.org/10.3390/molecules24030390
Ren X, Bao Y, Zhu Y, Liu S, Peng Z, Zhang Y, Zhou G. Isorhamnetin, Hispidulin, and Cirsimaritin Identified in Tamarix ramosissima Barks from Southern Xinjiang and Their Antioxidant and Antimicrobial Activities. Molecules. 2019; 24(3):390. https://doi.org/10.3390/molecules24030390
Chicago/Turabian StyleRen, Xiaopu, Yingjie Bao, Yuxia Zhu, Shixin Liu, Zengqi Peng, Yawei Zhang, and Guanghong Zhou. 2019. "Isorhamnetin, Hispidulin, and Cirsimaritin Identified in Tamarix ramosissima Barks from Southern Xinjiang and Their Antioxidant and Antimicrobial Activities" Molecules 24, no. 3: 390. https://doi.org/10.3390/molecules24030390