Pectin and Neutral Monosaccharides Production during the Simultaneous Hydrothermal Extraction of Waste Biomass from Refining of Sugar—Optimization with the Use of Doehlert Design
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition of Sugar Beet Pulp
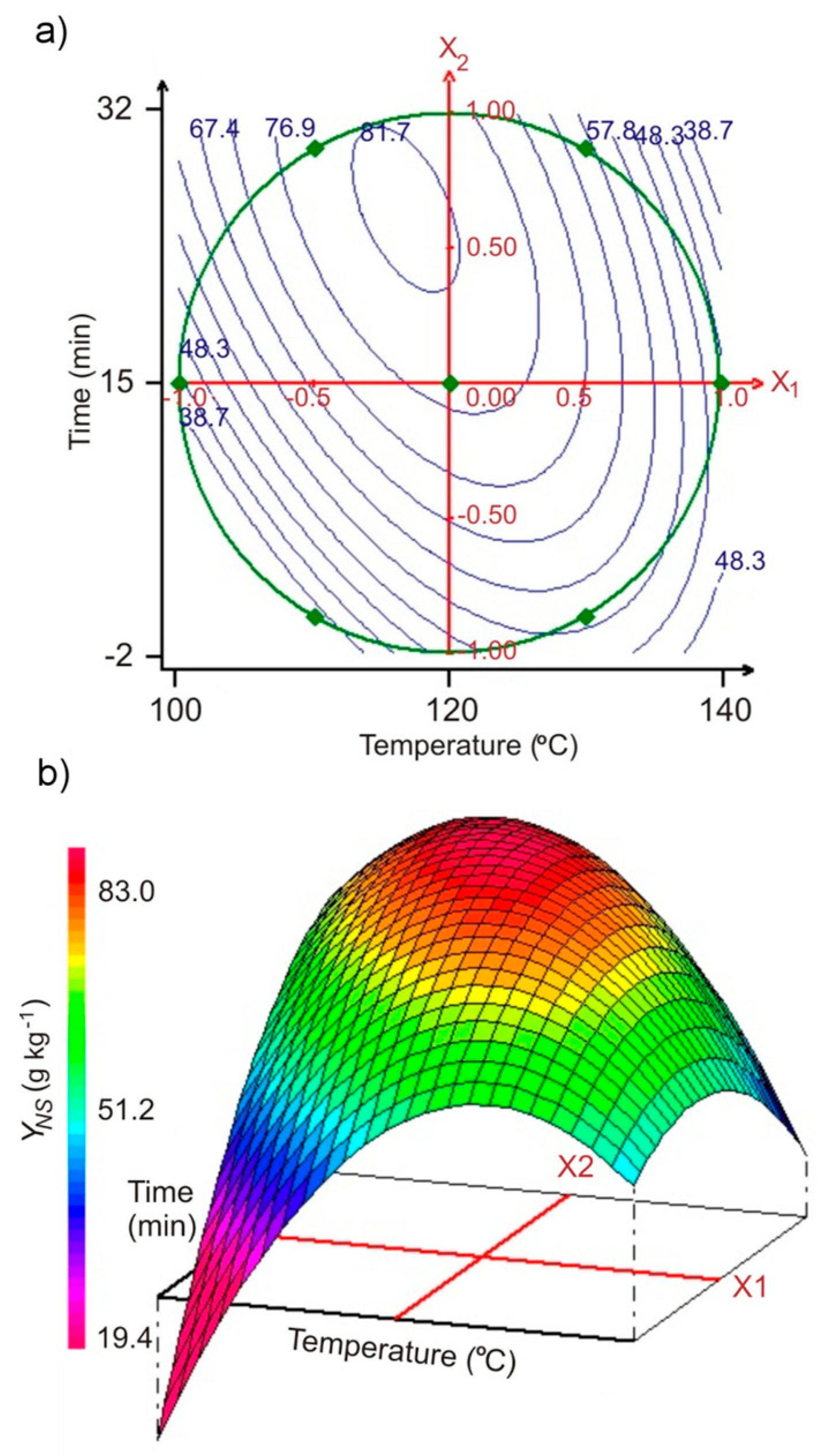
2.2. Modelling of Hydrothermal Extraction of Pectin from SBP Using Optimal Experimental Design
2.3. Characterization and Chemical Composition of Pectin
2.4. Modelling of Hydrothermolysis of Polysaccharide Components of SBP, Using Optimal Experimental Design
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Reactor and Experimental Procedure
3.3. Separation of Products of Pectin Hydrothermal Extraction from Sugar Beet Pulp
3.4. Analyses and Analytical Methods
3.5. Modelling and Optimization Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Olmos, J.C.; Hansen, M.Z. Enzymatic depolymerization of sugar beet pulp: Production and characterization of pectin and pectic-oligosaccharides as a potential source for functional carbohydrates. Chem. Eng. J. 2012, 192, 29–36. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry, catalysis and valorization of waste biomass. J. Mol. Catal. A Chem. 2016, 422, 3–12. [Google Scholar] [CrossRef]
- Knez, Ž.; Hrnčič, M.K.; Čolnik, M.; Škerget, M. Chemicals and value added compounds from biomass using sub- and supercritical water. J. Supercrit. Fluids 2018, 133, 591–602. [Google Scholar] [CrossRef]
- Kühnel, S.; Schols, H.A.; Gruppen, H. Aiming for the complete utilization of sugar-beet pulp: Examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol. Biofuels 2011, 4. [Google Scholar] [CrossRef]
- Micard, V.; Renard, C.M.G.C.; Thibault, J.F. Enzymatic saccharification of sugar-beet pulp. Enzyme Microb. Technol. 1996, 19, 162–170. [Google Scholar] [CrossRef]
- Sun, R.; Hughes, S. Extraction and physico-chemical characterization of pectins from sugar beet pulp. Polym. J. 1998, 30, 671–677. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Li, D.Q.; Du, G.M.; Jing, W.W.; Li, J.F.; Yan, J.Y.; Liu, Z.Y. Combined effects of independent variables on yield and protein content of pectin extracted from sugar beet pulp by citric acid. Carbohydr. Polym. 2015, 129, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Fu, X.; Abbasi, A.M.; Luo, Z.G. Preparation of environment-friendly pectin from sugar beet pulp and assessment of its emulsifying capacity. Int. J. Food Sci. Technol. 2015, 50, 1324–1330. [Google Scholar] [CrossRef]
- Chen, H.M.; Fu, X.; Luo, Z.G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. [Google Scholar] [CrossRef]
- Miyazawa, T.; Funazukuri, T. Hydrothermal production of mono(galacturonic acid) and the oligomers from poly(galacturonic acid) with water under pressures. Ind. Eng. Chem. Res. 2004, 43, 2310–2314. [Google Scholar] [CrossRef]
- Martínez, M.; Gullón, B.; Schols, H.A.; Alonso, J.L.; Parajó, J.C. Assessment of the production of oligomeric compounds from sugar beet pulp. Ind. Eng. Chem. Res. 2009, 48, 4681–4687. [Google Scholar] [CrossRef]
- Klinchongkon, K.; Khuwijitjaru, P.; Wiboonsirikul, J.; Adachi, S. Extraction of oligosaccharides from passion fruit peel by subcritical water treatment. J. Food Process. Eng. 2017, 40. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A. Extraction and rheological properties of pectin from fresh peach pomace. J. Food Eng. 1999, 39, 193–201. [Google Scholar] [CrossRef]
- Thibault, J.-F.; Renard, C.M.G.C.; Axelos, M.A.V.; Roger, P.; Crépeau, M.-J. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr. Res. 1993, 238, 271–286. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Zouambia, Y.; Moulai-Mostefa, N.; Krea, M. Structural characterization and surface activity of hydrophobically functionalized extracted pectins. Carbohydr. Polym. 2009, 78, 841–846. [Google Scholar] [CrossRef]
- Almohammed, F.; Koubaa, M.; Khelfa, A.; Nakaya, M.; Mhemdi, H.; Vorobiev, E. Pectin recovery from sugar beet pulp enhanced by high-voltage electrical discharges. Food Bioprod. Process. 2017, 103, 95–103. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Chen, F.; Lai, S.; Yang, H. Effects of vacuum impregnation with calcium ascorbate and disodium stannous citrate on Chinese red bayberry. Food Bioprocess. Technol. 2018, 11, 1300–1316. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Mustafa, M.A.; Misailidis, N.; Mateos-Salvador, F.; Du, C.; Campbell, G.M. Value analysis tool for feasibility studies of biorefineries integrated with value added production. Chem. Eng. Sci. 2008, 63, 503–519. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, F.; Zhang, P.; Lai, S.; Yang, H. Influence of rice bran wax coating on the physicochemical properties and pectin nanostructure of cherry tomatoes. Food Bioprocess. Technol. 2017, 10, 349–357. [Google Scholar] [CrossRef]
- Guo, X.; Meng, H.; Zhu, S.; Tang, Q.; Pan, R.; Yu, S. Stepwise ethanolic precipitation of sugar beet pectins from the acidic extract. Carbohydr. Polym. 2016, 136, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Y.; Mu, T.H.; Zhang, M.; Sun, H.N.; Chen, J.W.; Yu, M. Effects of pH and high hydrostatic pressure on the structural and rheological properties of sugar beet pectin. Food Hydrocoll. 2016, 60, 161–169. [Google Scholar] [CrossRef]
- Gharib-Bibalan, S. High Value-added products recovery from sugar processing by-products and residuals by green technologies: Opportunities, challenges, and prospects. Food Eng. Rev. 2018, 10, 95–111. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A.; Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, P.; Li, F.; Jin, S.; Wang, S.; Zhou, S. A review of lignocellulose change during hydrothermal pretreatment for bioenergy production. Curr. Org. Chem. 2016, 20, 2799–2809. [Google Scholar] [CrossRef]
- Levigne, S.; Ralet, M.-C.; Thibault, J.-F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr. Polym. 2002, 49, 145–153. [Google Scholar] [CrossRef]
- Pińkowska, H.; Wolak, P.; Oliveros, E. Production of xylose and glucose from rapeseed straw in subcritical water—Use of Doehlert design for optimizing the reaction conditions. Biomass Bioenergy 2013, 58, 188–197. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Hames, B.; Scarlata, C.; Sluiter, A. Determination of Protein Content in Biomass; Laboratory Analytical Procedure (LAP) Technical Report NREL/TP-510-42625; Golden, CO, USA, 2008. [Google Scholar]
- Undersander, D.; Mertens, D.; Thiex, N. Forage analyses procedures. Natl. Forage Test. Assoc. 1993, 68137, 1–139. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Schols, H.A.; Pilnik, W. Determination of the degree of methylation and acetylation of pectins by h.p.l.c. Top. Catal. 1986, 1, 65–70. [Google Scholar] [CrossRef]
- Atkinson, A.C.; Donev, A.N. Optimum Experimental Designs; Clarendon Press: Oxford, UK, 1992; ISBN 9780198522546. [Google Scholar]
- Ferreira, S.L.C.; Dos Santos, W.N.L.; Quintella, C.M.; Neto, B.B.; Bosque-Sendra, J.M. Doehlert matrix: A chemometric tool for analytical chemistry—Review. Talanta 2004, 63, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- NEMROD, Version 2002, LPRAI, B.P. no. 7, 13311 Marseille Cédex 14, France. Available online: www.nemrodw.com (accessed on 29 September 2016).
Sample Availability: Samples of the compounds are available from the authors |

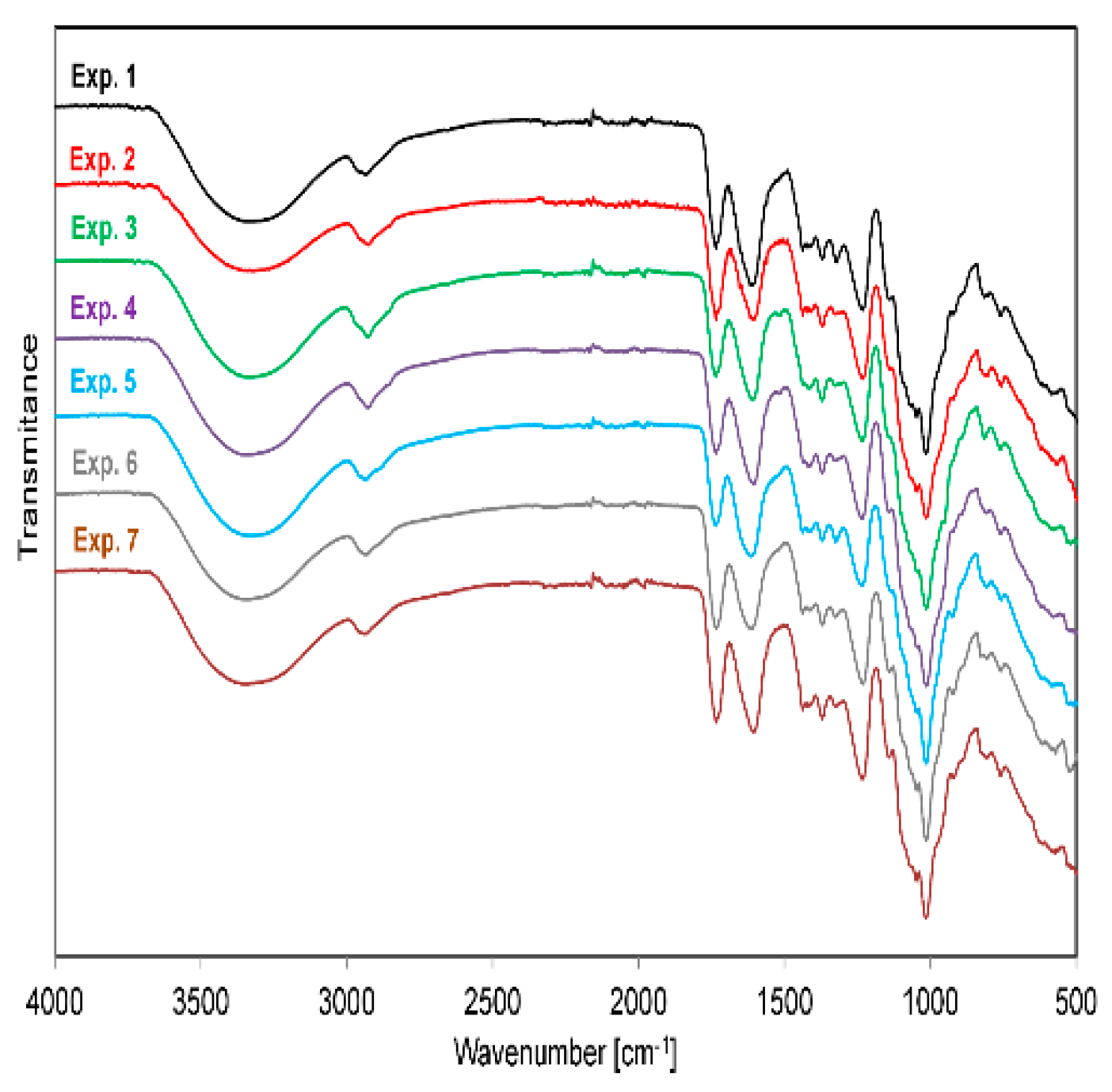
| Component | Content (g kg−1) |
|---|---|
| Dry matter | 210.1 ± 1.2* |
| Ash | 23.7 ± 0.4 |
| Total protein (N∙6.25) | 88.4 ± 0.5 |
| Diethyl ether extractable substance | 55.2 ± 0.5 |
| Pectin | 134.8 ± 0.8 |
| Hemicellulose | 272.3 ± 2.4 |
| Cellulose | 140.0 ± 2.3 |
| Lignin | 88.6 ± 3.4 |
| Remaining sucrose as saccharide after inversion | 11.2 ± 1.2 |
| Monosaccharides **: | |
| Arabinose | 190.4 ± 0.5 |
| Xylose | 16.6 ± 1.0 |
| Glucose | 189.3 ± 0.9 |
| Sum of galactose, mannose and rhamnose | 80.1 ± 2.8 |
| Uronic acids | 188.3 ± 1.3 |
| Acetic acid | 1.7 ± 0.9 |
| Methanol | 4.2 ± 0.9 |
| Pectin | Sum of Neutral Monosaccharides | |||||||
|---|---|---|---|---|---|---|---|---|
| Experiment | x1 | x2 | u1 T (°C) | u2 t (min) | YP-exp. ** (g kg−1) | YP-cal. *** (g kg−1) | YNS-exp. ** (g kg−1) | YNS-cal. *** (g kg−1) |
| 1 | +1 | 0 | 140 | 15.0 | 88.1 | 93.0 | 52.3 | 49.3 |
| 2 | −1 | 0 | 100 | 15.0 | 91.1 | 86.2 | 38.7 | 41.8 |
| 3 | +0.5 | +0.866 | 130 | 30.0 | 102.5 | 97.6 | 61.1 | 64.2 |
| 4 | −0.5 | −0.866 | 110 | 0.0 | 57.3 | 62.2 | 38.9 | 35.8 |
| 5 | +0.5 | −0.866 | 130 | 0.0 | 89.4 | 84.5 | 55.9 | 59.0 |
| 6 | −0.5 | +0.866 | 110 | 30.0 | 108.9 | 113.2 | 83.0 | 79.9 |
| 7 | 0 | 0 | 120 | 15.0 | 118.9 | 118.0 | 78.9 | 78.4 |
| 7’ * | 0 | 0 | 120 | 15.0 | 117.9 | 118.0 | 77.0 | 78.4 |
| 7’’ * | 0 | 0 | 120 | 15.0 | 117.2 | 118.0 | 79.2 | 78.4 |
| Experiment | Uronic Acids (g kg−1) | Arabinose (g kg−1) | Glucose (g kg−1) | * (g kg−1) | Xylose (g kg−1) | DM ** (% mol) | DA *** (% mol) |
|---|---|---|---|---|---|---|---|
| 1 | 499.2 | 131.6 | 11.5 | 181.3 | 12.5 | 31.9 | 16.1 |
| 2 | 506.1 | 119.6 | 9.4 | 152.9 | 11.1 | 54.2 | 18.0 |
| 3 | 499.0 | 133.0 | 13.4 | 171.9 | 15.1 | 39.2 | 13.6 |
| 4 | 503.9 | 114.4 | 9.5 | 118.1 | 12.4 | 53.2 | 21.6 |
| 5 | 504.2 | 121.6 | 9.5 | 157.2 | 13.7 | 45.0 | 21.3 |
| 6 | 499.9 | 121.9 | 9.3 | 160.6 | 13.9 | 47.2 | 13.8 |
| 7 | 511.4 | 122.4 | 9.4 | 165.1 | 13.4 | 51.9 | 16.9 |
| 7’ | 513.4 | 123.1 | 9.7 | 165.9 | 12.9 | 52.0 | 16.8 |
| 7’’ | 513.7 | 122.9 | 9.9 | 165.1 | 12.2 | 51.7 | 16.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pińkowska, H.; Krzywonos, M.; Wolak, P.; Złocińska, A. Pectin and Neutral Monosaccharides Production during the Simultaneous Hydrothermal Extraction of Waste Biomass from Refining of Sugar—Optimization with the Use of Doehlert Design. Molecules 2019, 24, 472. https://doi.org/10.3390/molecules24030472
Pińkowska H, Krzywonos M, Wolak P, Złocińska A. Pectin and Neutral Monosaccharides Production during the Simultaneous Hydrothermal Extraction of Waste Biomass from Refining of Sugar—Optimization with the Use of Doehlert Design. Molecules. 2019; 24(3):472. https://doi.org/10.3390/molecules24030472
Chicago/Turabian StylePińkowska, Hanna, Małgorzata Krzywonos, Paweł Wolak, and Adrianna Złocińska. 2019. "Pectin and Neutral Monosaccharides Production during the Simultaneous Hydrothermal Extraction of Waste Biomass from Refining of Sugar—Optimization with the Use of Doehlert Design" Molecules 24, no. 3: 472. https://doi.org/10.3390/molecules24030472







