Probing Coherent Vibrations of Organic Phosphonate Radical Cations with Femtosecond Time-Resolved Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Femtosecond Time-Resolved Mass Spectrometry (FTRMS)
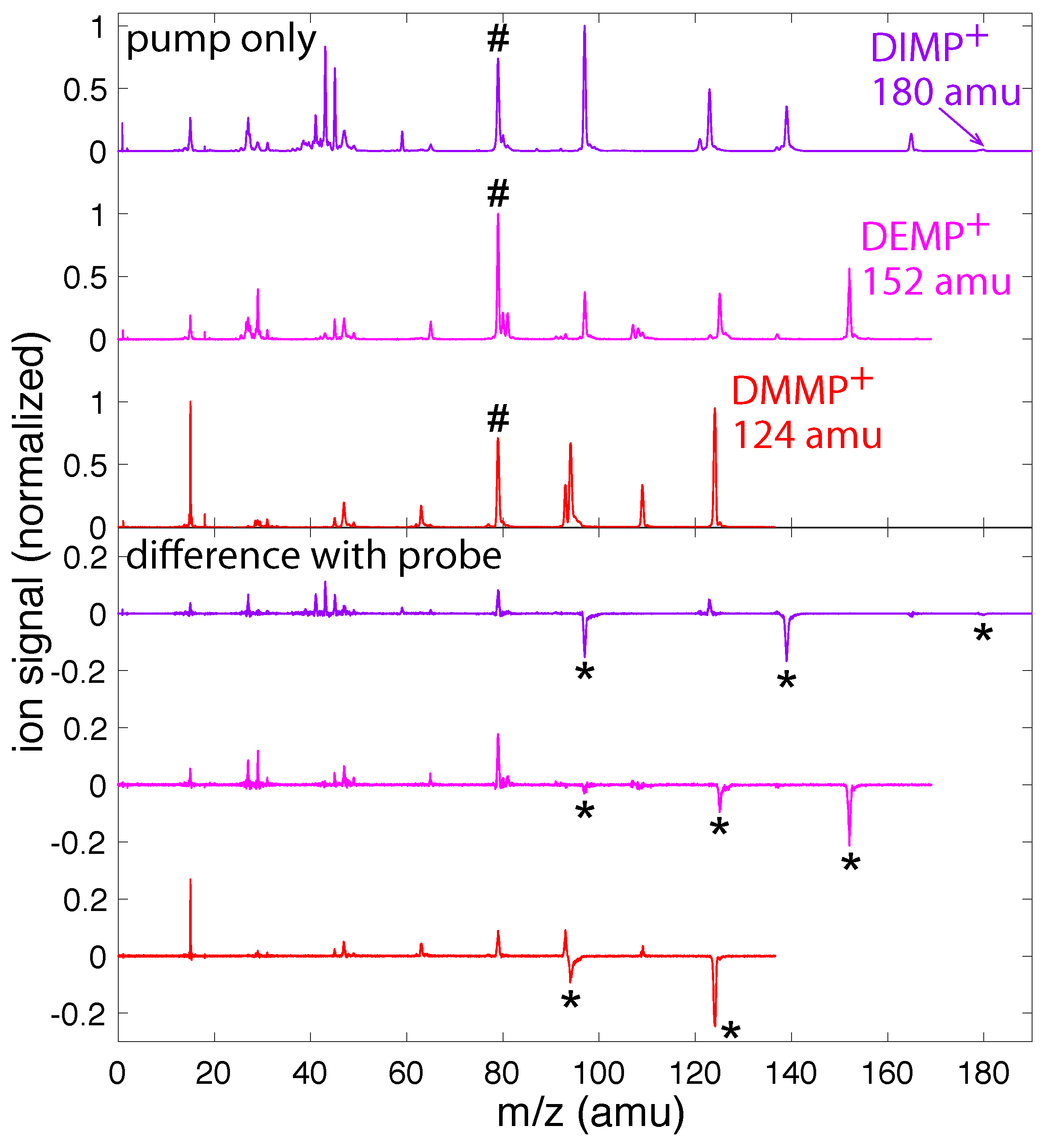
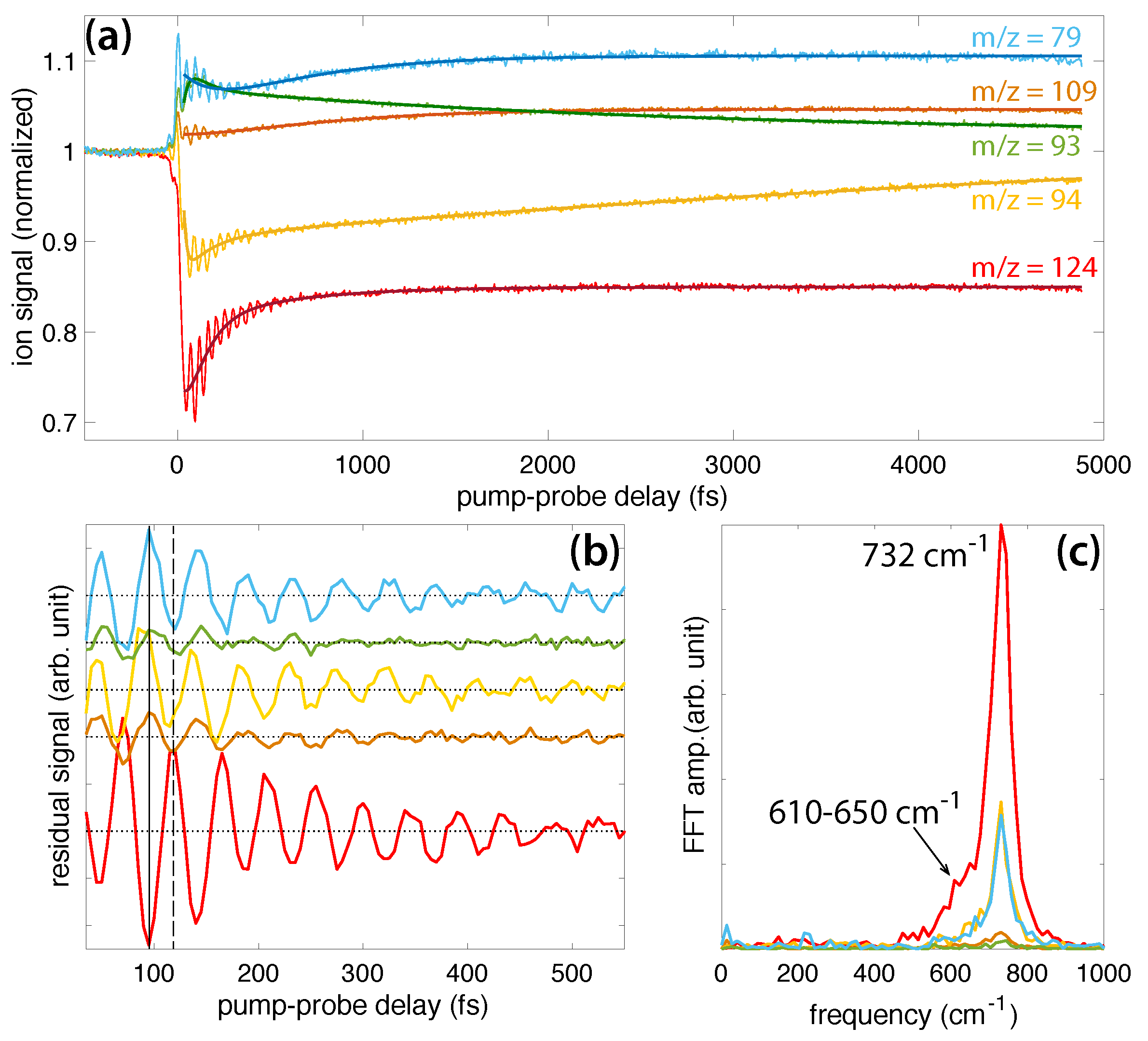
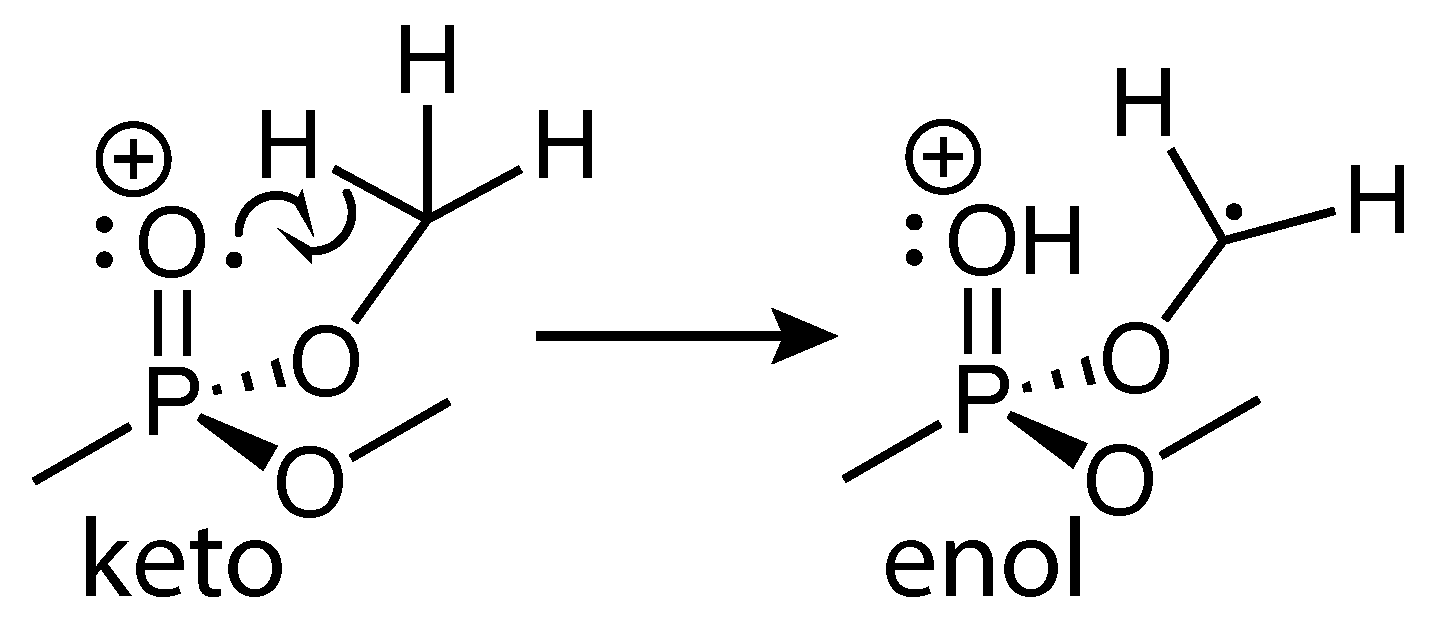
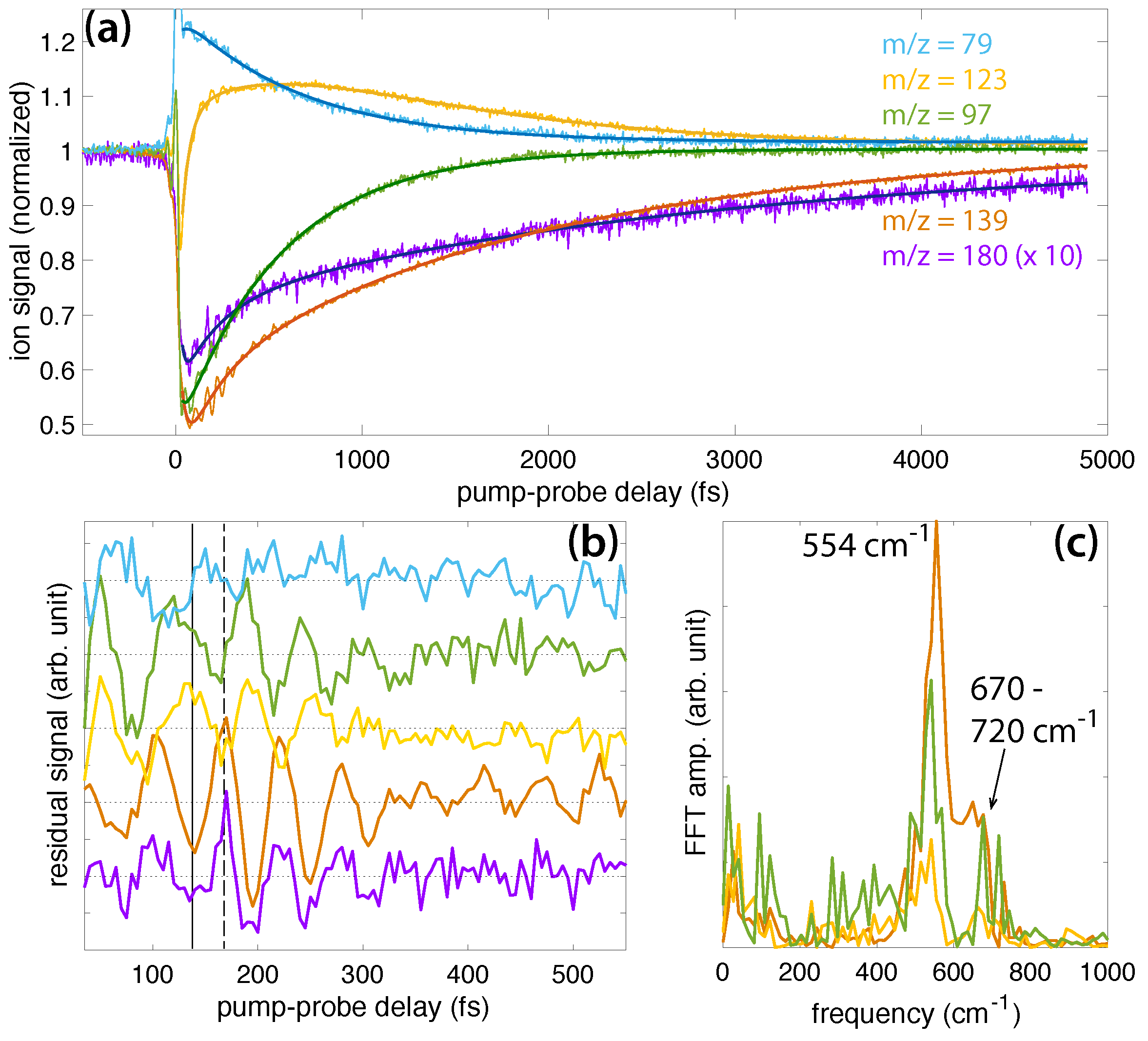
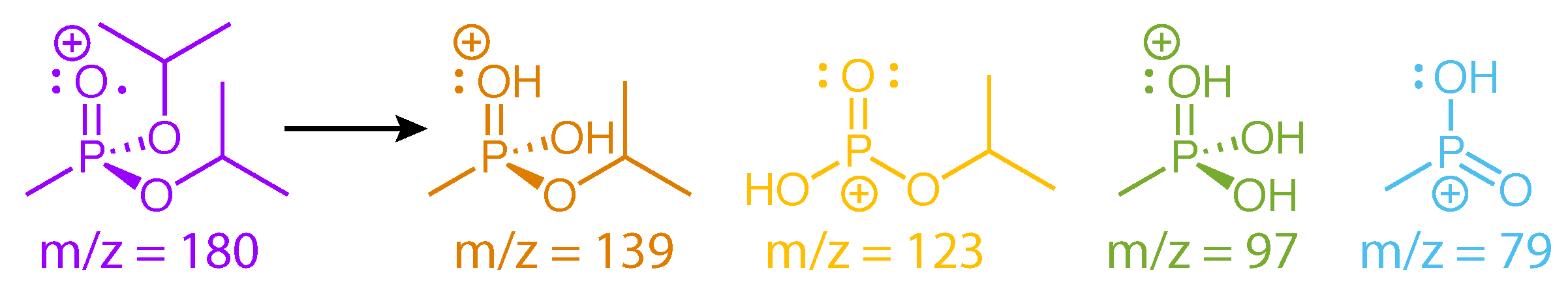
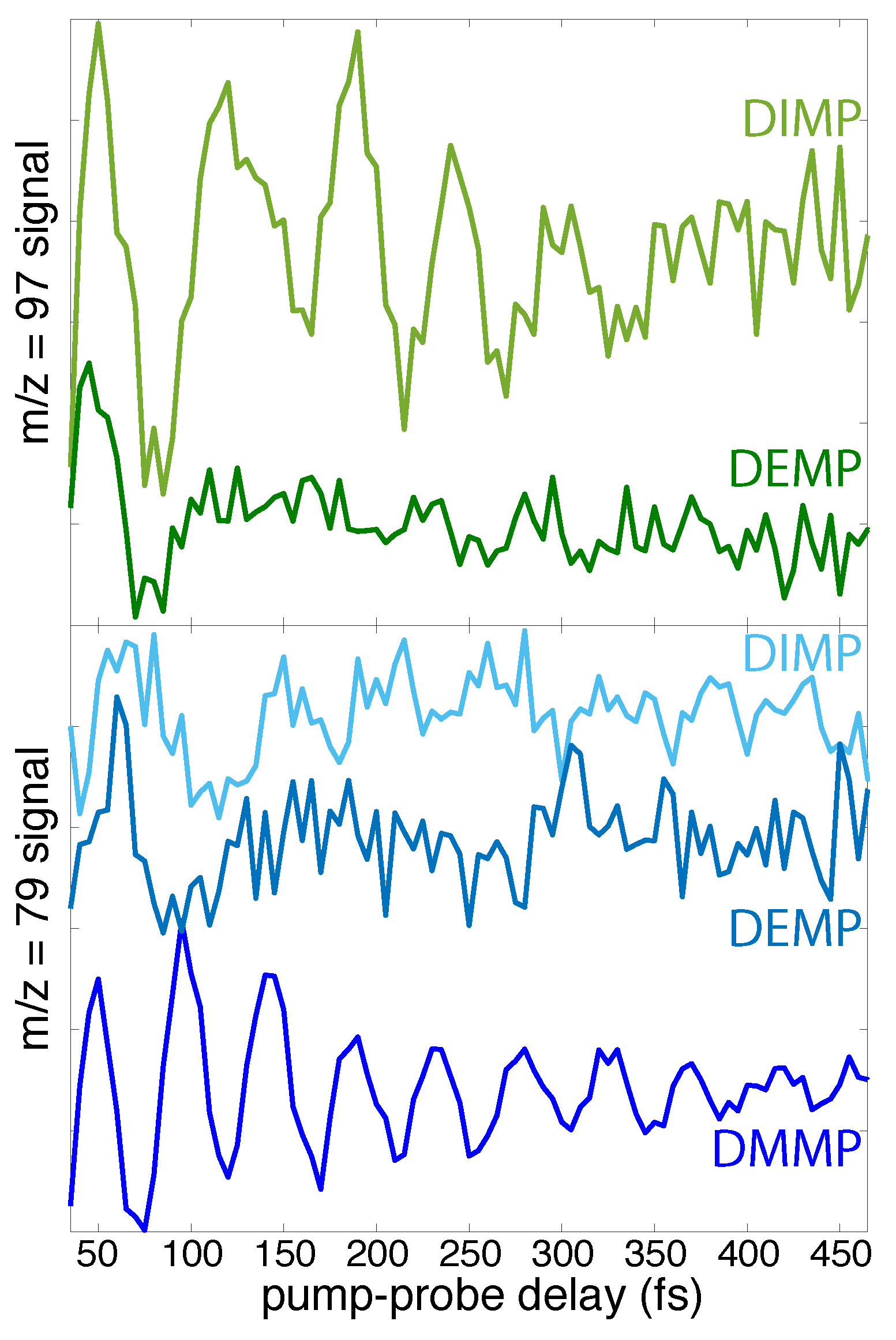
2.1.1. DMMP
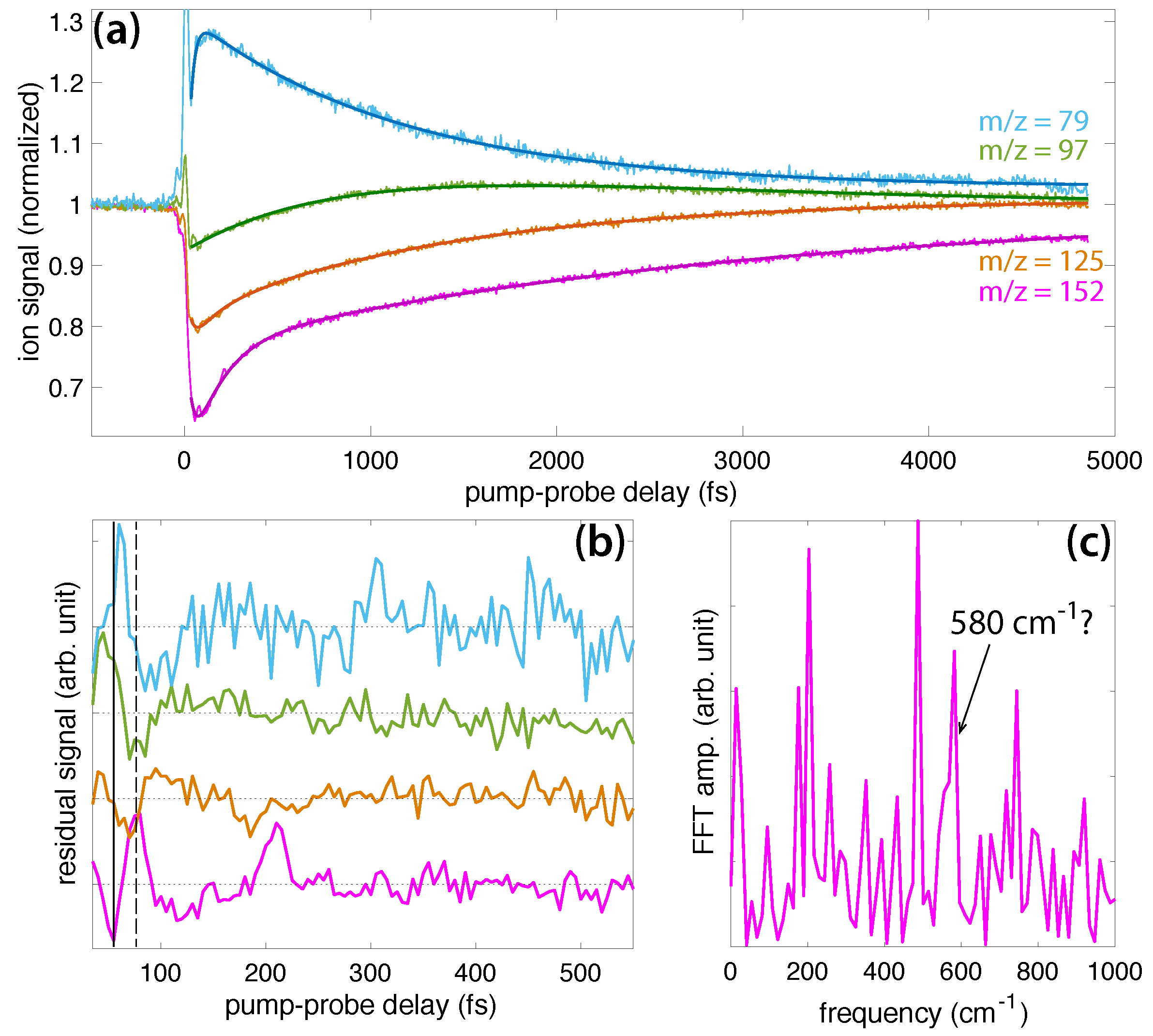
2.1.2. DEMP
2.1.3. DIMP
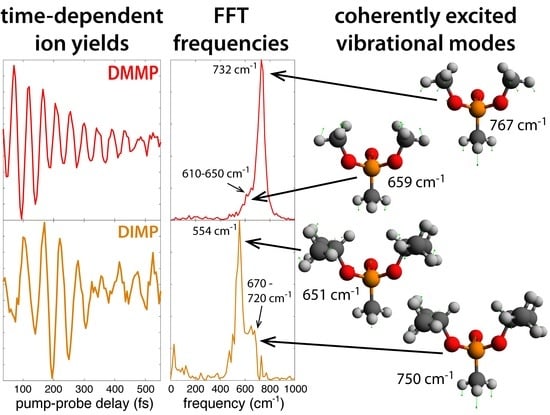
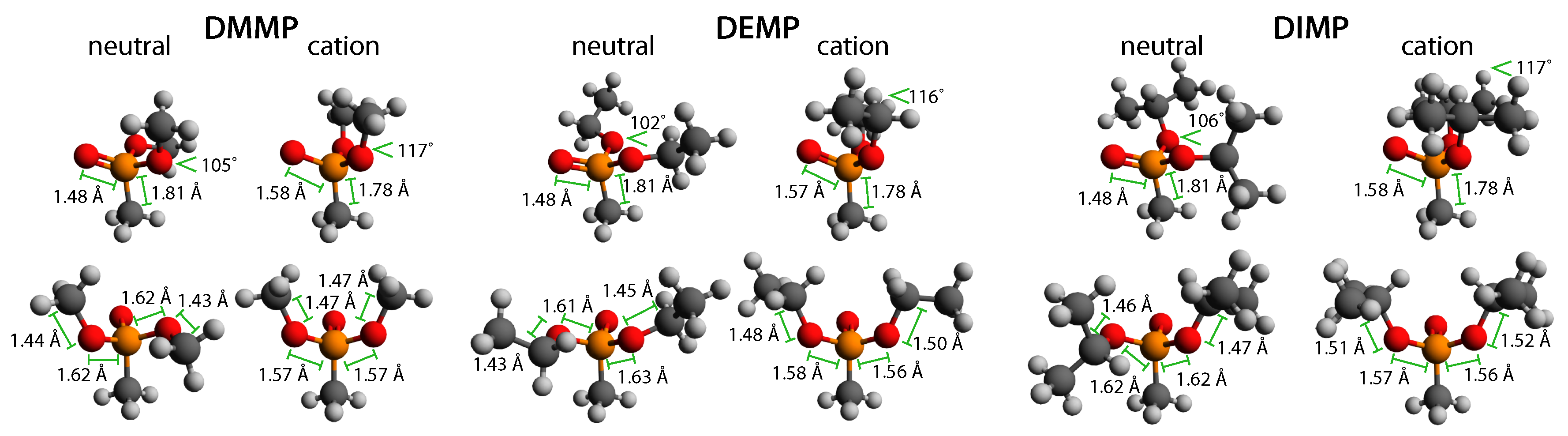
2.2. Assignments of Coherently Excited Vibrational Modes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.3. Theoretical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FTRMS | Femtosecond time-resolved mass spectrometry |
| DNA | Deoxyribonucleic acid |
| DMMP | Dimethyl methylphosphonate |
| DEMP | Diethyl methylphosphonate |
| DIMP | Diisopropyl methylphosphonate |
| FFT | Fast Fourier transform |
References
- Sevilla, M.D.; Becker, D.; Kumar, A.; Adhikary, A. Gamma and ion-beam irradiation of DNA: Free radical mechanisms, electron effects, and radiation chemical track structure. Radiat. Phys. Chem. 2016, 128, 60–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikary, A.; Becker, D.; Palmer, B.J.; Heizer, A.N.; Sevilla, M.D. Direct Formation of the C5’-Radical in the Sugar–Phosphate Backbone of DNA by High-Energy Radiation. J. Phys. Chem. B 2012, 116, 5900–5906. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Marignier, J.L.; Pernot, P.; Houee-Levin, C.; Kumar, A.; Sevilla, M.D.; Adhikary, A.; Mostafavi, M. Direct observation of the oxidation of DNA bases by phosphate radicals formed under radiation: A model of the backbone-to-base hole transfer. Phys. Chem. Chem. Phys. 2018, 20, 14927–14937. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Denisov, S.A.; Marignier, J.L.; Pernot, P.; Adhikary, A.; Seki, S.; Mostafavi, M. Ultrafast Electron Attachment and Hole Transfer Following Ionizing Radiation of Aqueous Uridine Monophosphate. J. Phys. Chem. Lett. 2018, 9, 5105–5109. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.L. Measurement of oxidatively generated base damage in cellular DNA. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 711, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zewail, A.H. Femtochemistry: Atomic-Scale Dynamics of the Chemical Bond. J. Phys. Chem. A 2000, 104, 5660–5694. [Google Scholar] [CrossRef]
- Pearson, B.; Nichols, S.; Weinacht, T. Molecular Fragmentation Driven by Ultrafast Dynamic Ionic Resonances. J. Chem. Phys. 2007, 127, 131101. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.; Weinacht, T.; Rozgonyi, T.; Pearson, B. Strong-Field Phase-Dependent Molecular Dissociation. Phys. Rev. A 2009, 79, 043407. [Google Scholar] [CrossRef]
- Geißler, D.; Rozgonyi, T.; Gonzalez-Vazquez, J.; Gonzalez, L.; Nichols, S.; Weinacht, T. Creation of Multihole Molecular Wave Packets via Strong-Field Ionization. Phys. Rev. A 2010, 82, 011402. [Google Scholar] [CrossRef]
- Brogaard, R.Y.; Møller, K.B.; Sølling, T.I. Real-Time Probing of Structural Dynamics by Interaction between Chromophores. J. Phys. Chem. A 2011, 115, 12120–12125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.W.; Chen, W.K.; Cheng, P.Y. Femtosecond Pump-Probe Photoionization-Photofragmentation Spectroscopy: Photoionization-Induced Twisting and Coherent Vibrational Motion of Azobenzene Cation. J. Chem. Phys. 2009, 131, 134308. [Google Scholar] [CrossRef] [PubMed]
- Munkerup, K.; Romanov, D.; Bohinski, T.; Stephansen, A.B.; Levis, R.J.; Sølling, T.I. Conserving Coherence and Storing Energy during Internal Conversion: Photoinduced Dynamics of cis- and trans-Azobenzene Radical Cations. J. Phys. Chem. A 2017, 121, 8642–8651. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lozovoy, V.; Shah, J.; Dantus, M. Photodissociation Dynamics of Acetophenone and Its Derivatives with Intense Nonresonant Femtosecond Pulses. J. Phys. Chem. A 2011, 115, 1305–1312. [Google Scholar] [CrossRef]
- Konar, A.; Shu, I.; Lozovoy, V.V.; Jackson, J.E.; Levine, B.G.; Dantus, M. Polyatomic Molecules under Intense Femtosecond Laser Irradiation. J. Phys. Chem. A 2014, 118, 11433–11450. [Google Scholar] [CrossRef] [PubMed]
- Bohinski, T.; Tibbetts, K.M.; Tarazkar, M.; Romanov, D.A.; Matsika, S.; Levis, R.J. Strong Field Adiabatic Ionization Prepares a Launch State for Coherent Control. J. Phys. Chem. Lett. 2014, 5, 4305–4309. [Google Scholar] [CrossRef]
- Tibbetts, K.M.; Tarazkar, M.; Bohinski, T.; Romanov, D.A.; Matsika, S.; Levis, R.J. Controlling the Dissociation Dynamics of Acetophenone Radical Cation Through Excitation of Ground and Excited State Wavepackets. J. Phys. B At. Opt. Mol. Phys. 2015, 48, 164002. [Google Scholar] [CrossRef]
- Ampadu Boateng, D.; Gutsev, G.L.; Jena, P.; Tibbetts, K.M. Dissociation dynamics of 3- and 4-nitrotoluene radical cations: Coherently driven C–NO2 bond homolysis. J. Chem. Phys. 2018, 148, 134305. [Google Scholar] [CrossRef] [Green Version]
- Ampadu Boateng, D.; Gutsev, G.L.; Jena, P.; Tibbetts, K.M. Ultrafast coherent vibrational dynamics in dimethyl methylphosphonate radical cation. Phys. Chem. Chem. Phys. 2018, 20, 4636–4640. [Google Scholar] [CrossRef]
- Ampadu Boateng, D.; Word, M.D.; Gutsev, L.G.; Jena, P.; Tibbetts, K.M. Conserved Vibrational Coherence in the Ultrafast Rearrangement of 2-Nitrotoluene Radical Cation. J. Phys. Chem. A 2019. [Google Scholar] [CrossRef]
- Roberts, G.M.; Marroux, H.J.B.; Grubb, M.P.; Ashfold, M.N.R.; Orr-Ewing, A.J. On the Participation of Photoinduced N–H Bond Fission in Aqueous Adenine at 266 and 220 nm: A Combined Ultrafast Transient Electronic and Vibrational Absorption Spectroscopy Study. J. Phys. Chem. A 2014, 118, 11211–11225. [Google Scholar] [CrossRef] [Green Version]
- Stavros, V.G.; Verlet, J.R. Gas-Phase Femtosecond Particle Spectroscopy: A Bottom-Up Approach to Nucleotide Dynamics. Annu. Rev. Phys. Chem. 2016, 67, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Horsman, G.P.; Zechel, D.L. Phosphonate Biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef] [PubMed]
- Bafus, D.A.; Gallegos, E.J.; Kiser, R.W. An Electron Impact Investigation of Some Alkyl Phosphate Esters. J. Phys. Chem. 1966, 70, 2614–2619. [Google Scholar] [CrossRef]
- Sass, S.; Fisher, T.L. Chemical ionization and electron impact mass spectrometry of some organophosphonate compounds. Org. Mass Spectrom. 1979, 14, 257–264. [Google Scholar] [CrossRef]
- Holtzclaw, J.R.; Wyatt, J.R.; Campana, J.E. Structure and Fragmentation of Dimethyl Methylphosphonate and Trimethyl Phosphite. Organ. Mass Spectrom. 1985, 20, 90–97. [Google Scholar] [CrossRef]
- Holtzclaw, J.R.; Wyatt, J.R. Keto-to-Enol Isomerization in the Molecular Ion of Dimethylmethylphosphonate. Organ. Mass Spectrom. 1988, 23, 261–266. [Google Scholar] [CrossRef]
- Zeller, L.; Farrell, J.; Kenttamaa, H.I.; Vainiotalo, P. Long-lived radical cations of simple organophosphates isomerize spontaneously to distonic structures in the gas phase. J. Am. Chem. Soc. 1992, 114, 1205–1214. [Google Scholar] [CrossRef]
- Bell, A.; Despeyroux, D.; Murrell, J.; Watts, P. Fragmentation and reactions of organophosphate ions produced by electrospray ionization. Int. J. Mass Spectrom. Ion Process. 1997, 165–166, 533–550. [Google Scholar] [CrossRef]
- Groenewold, G.S.; Scott, J.R.; Lee, E.D.; Lammert, S.A. Rapid analysis of organophosphonate compounds recovered from vinyl floor tile using vacuum extraction coupled with a fast-duty cycle GC/MS. Anal. Methods 2013, 5, 2227–2236. [Google Scholar] [CrossRef]
- Liang, S.; Hemberger, P.; Neisius, N.M.; Bodi, A.; Grützmacher, H.; Levalois-Grützmacher, J.; Gaan, S. Elucidating the Thermal Decomposition of Dimethyl Methylphosphonate by Vacuum Ultraviolet (VUV) Photoionization: Pathways to the PO Radical, a Key Species in Flame-Retardant Mechanisms. Chem. Eur. J. 2015, 21, 1073–1080. [Google Scholar] [CrossRef]
- Gutsev, G.L.; Ampadu Boateng, D.; Jena, P.; Tibbetts, K.M. A Theoretical and Mass Spectrometry Study of Dimethyl Methylphosphonate: New Isomers and Cation Decay Channels in an Intense Femtosecond Laser Field. J. Phys. Chem. A 2017, 121, 8414–8424. [Google Scholar] [CrossRef] [PubMed]
- McLafferty, F.W. Mass Spectrometric Analysis. Molecular Rearrangements. Anal. Chem. 1959, 31, 82–87. [Google Scholar] [CrossRef]
- Cuisset, A.; Mouret, G.; Pirali, O.; Roy, P.; Cazier, F.; Nouali, H.; Demaison, J. Gas-Phase Vibrational Spectroscopy and Ab Initio Study of Organophosphorous Compounds: Discrimination between Species and Conformers. J. Phys. Chem. B 2008, 112, 12516–12525. [Google Scholar] [CrossRef]
- Meyrick, C.I.; Thompson, H.W. 53. Vibrational spectra of alkyl esters of phosphorus oxy-acids. J. Chem. Soc. 1950, 225–229. [Google Scholar] [CrossRef]
- Maarsen, J.W.; Smit, M.C.; Matze, J. The Raman and infra-red spectra of some compounds (iH7C3O)2PXO. Recueil des Travaux Chimiques des Pays-Bas 1957, 76, 713–723. [Google Scholar] [CrossRef]
- Veken, B.J.V.D.; Herman, M.A. Vibrational Spectra of ch3po(och3)2 and Isotopically Substituted Derivatives. Phosphorus Sulfur Relat. Elem. 1981, 10, 357–367. [Google Scholar] [CrossRef]
- NIST Standard Reference Database 69. Available online: http://webbook.nist.gov/chemistry/ (accessed on 15 December 2018).
- Mott, A.J.; Rez, P. Calculated infrared spectra of nerve agents and simulants. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 91, 256–260. [Google Scholar] [CrossRef]
- Hameka, H.F.; Carrieri, A.H.; Jensen, J.O. Calculations of the structure and the vibrational infrared frequencies of some methylphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 1992, 66, 1–11. [Google Scholar] [CrossRef]
- Lezius, M.; Blanchet, V.; Ivanov, M.Y.; Stolow, A. Polyatomic Molecules in Strong Laser Fields: Nonadiabatic Multielectron Dynamics. J. Chem. Phys. 2002, 117, 1575–1588. [Google Scholar] [CrossRef]
- Cui, Y.; Bhardwaj, C.; Milasinovic, S.; Carlson, R.P.; Gordon, R.J.; Hanley, L. Molecular Imaging and Depth Profiling of Biomaterials Interfaces by Femtosecond Laser Desorption Postionization Mass Spectrometry. ACS Appl. Mater. Interfaces 2013, 5, 9269–9275. [Google Scholar] [CrossRef]
- Cui, Y.; Veryovkin, I.V.; Majeski, M.W.; Cavazos, D.R.; Hanley, L. High Lateral Resolution vs Molecular Preservation in near-IR fs-Laser Desorption Postionization Mass Spectrometry. Anal. Chem. 2015, 87, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hu, Y.; Chen, J.; Jin, S. Laser Desorption Postionization Mass Spectrometry Imaging of Folic Acid Molecules in Tumor Tissue. Anal. Chem. 2017, 89, 8238–8243. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Y.; Lu, Q.; Wang, P.; Zhan, H. Molecular imaging of small molecule drugs in animal tissues using laser desorption postionization mass spectrometry. Analyst 2017, 142, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.V.; Gelb, L.D.; Barry, G.E.; Subanajouy, P.; Poudel, A.; Hara, M.; Veryovkin, I.V.; Bell, G.I.; Hanley, L. Femtosecond laser desorption ionization mass spectrometry imaging and multivariate analysis of lipids in pancreatic tissue. Biointerphases 2018, 13, 03B416. [Google Scholar] [CrossRef] [PubMed]
- Ampadu Boateng, D.; Tibbetts, K.M. Measurement of Ultrafast Vibrational Coherences in Polyatomic Radical Cations with Strong-Field Adiabatic Ionization. JoVE 2018, 138, e58263. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.J.; Trebino, R. Characterization of Arbitrary Femtosecond Pulses Using Frequency-Resolved Optical Gating. IEEE J. Quant. Electron. 1993, 29, 571–579. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01. 2016. Available online: http://gaussian.com/citation/ (accessed on 31 January 2019).
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Bolton, E.; Wang, Y.; Thiessen, P.; Bryant, S. PubChem. Integrated platform of small molecules and biological activities. In Annual Reports in Computational Chemistry; American Chemical Society: Washington, DC, USA, 2008. [Google Scholar]
- Zhurko, G.A. Chemcraft: Graphical Program for Visualization of Quantum Chemistry Computations. Available online: https://chemcraftprog.com/ (accessed on 10 January 2019).
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

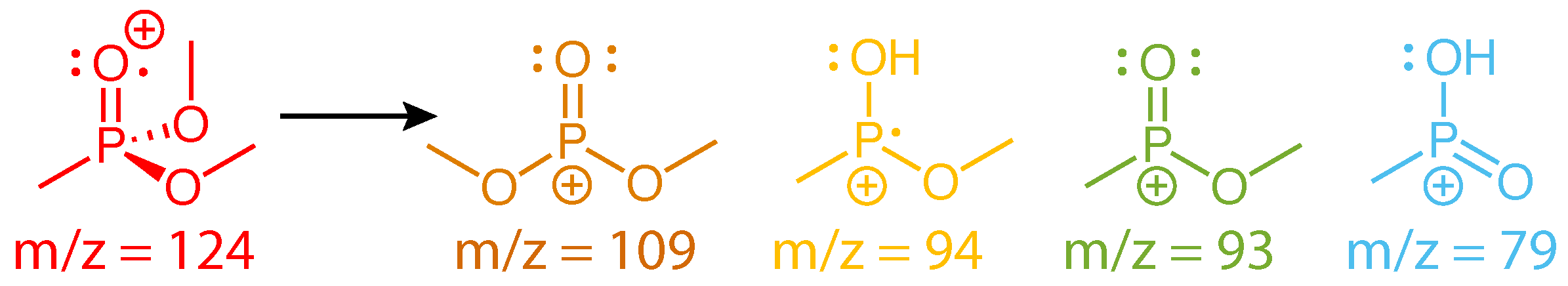
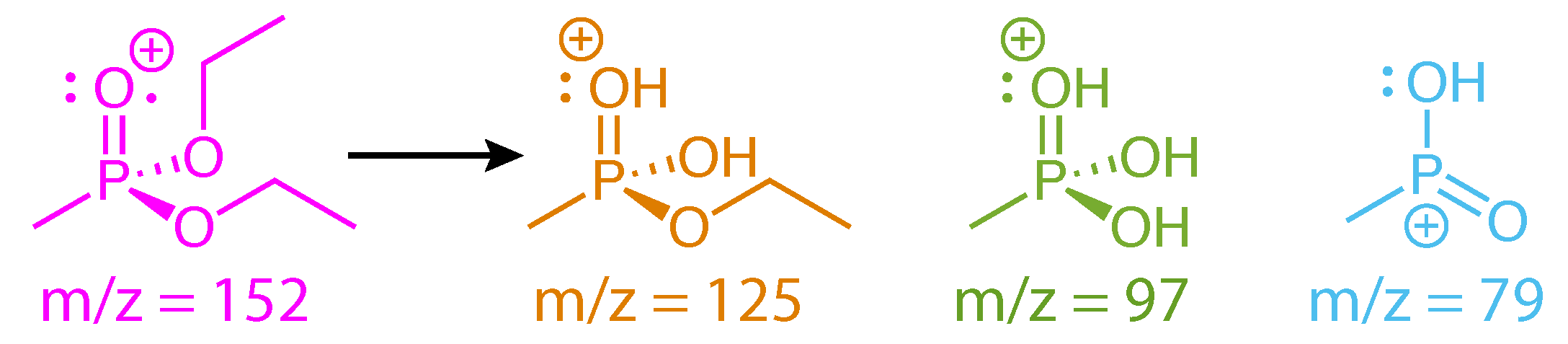


| Molecule | Neutral E (Hartree) | Relaxed Cation E (Hartree) | IPvert (eV) | Erelax (eV) |
|---|---|---|---|---|
| DMMP | −686.8138 | −686.4663 | 10.31 | 0.66 |
| DEMP | −765.4112 | −765.0739 | 9.82 | 0.64 |
| DIMP | −844.0129 | −843.6786 | 9.55 | 0.58 |
| Mode | DMMP Neutral | Cation | DEMP Neutral | Cation | DIMP Neutral | Cation |
|---|---|---|---|---|---|---|
| A | 672 (712) | 659 | 689 (715) | 656 | 690 (719) | 651 |
| B | 759 (786) | 761 | 765 (771) | 711 | 742 (748) | 665 |
| C | 794 (818) | 767 | 788 (806) | 756 | 778 (791) | 750 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ampadu Boateng, D.; Word, M.D.; Tibbetts, K.M. Probing Coherent Vibrations of Organic Phosphonate Radical Cations with Femtosecond Time-Resolved Mass Spectrometry. Molecules 2019, 24, 509. https://doi.org/10.3390/molecules24030509
Ampadu Boateng D, Word MD, Tibbetts KM. Probing Coherent Vibrations of Organic Phosphonate Radical Cations with Femtosecond Time-Resolved Mass Spectrometry. Molecules. 2019; 24(3):509. https://doi.org/10.3390/molecules24030509
Chicago/Turabian StyleAmpadu Boateng, Derrick, Mi’Kayla D. Word, and Katharine Moore Tibbetts. 2019. "Probing Coherent Vibrations of Organic Phosphonate Radical Cations with Femtosecond Time-Resolved Mass Spectrometry" Molecules 24, no. 3: 509. https://doi.org/10.3390/molecules24030509