The Bispidinone Derivative 3,7-Bis-[2-(S)-amino-3-(1H-indol-3-yl)-propionyl]-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one Dihydrochloride Induces an Apoptosis-Mediated Cytotoxic Effect on Pancreatic Cancer Cells In Vitro
Abstract
:1. Introduction
2. Results
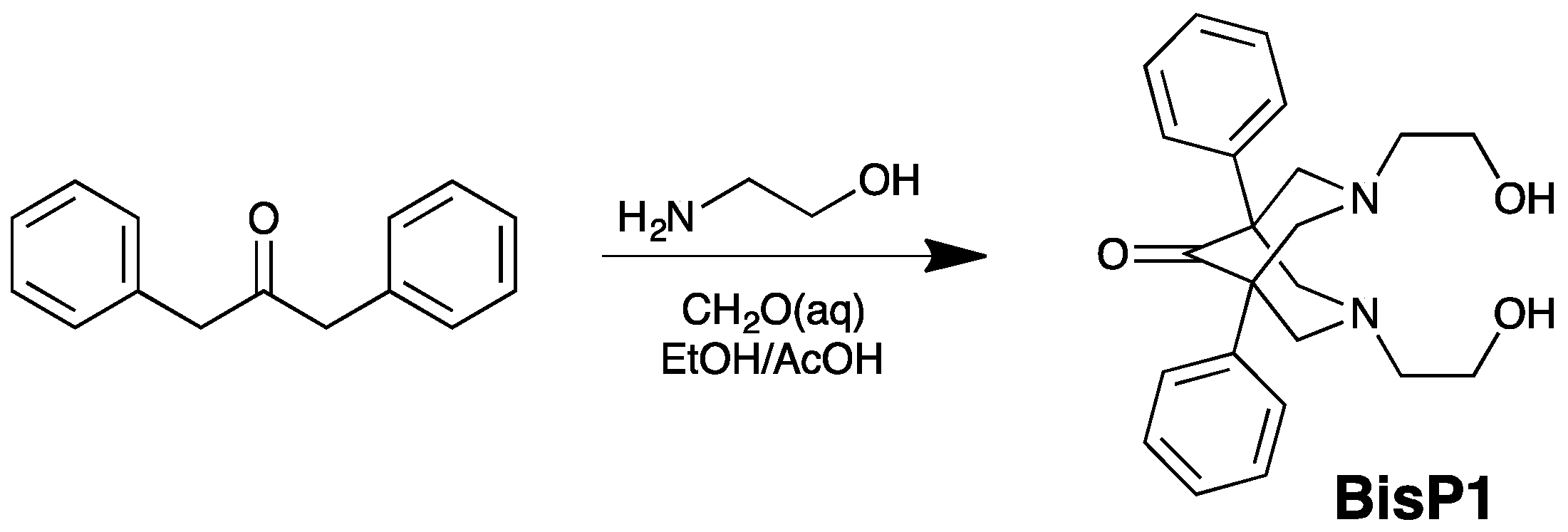
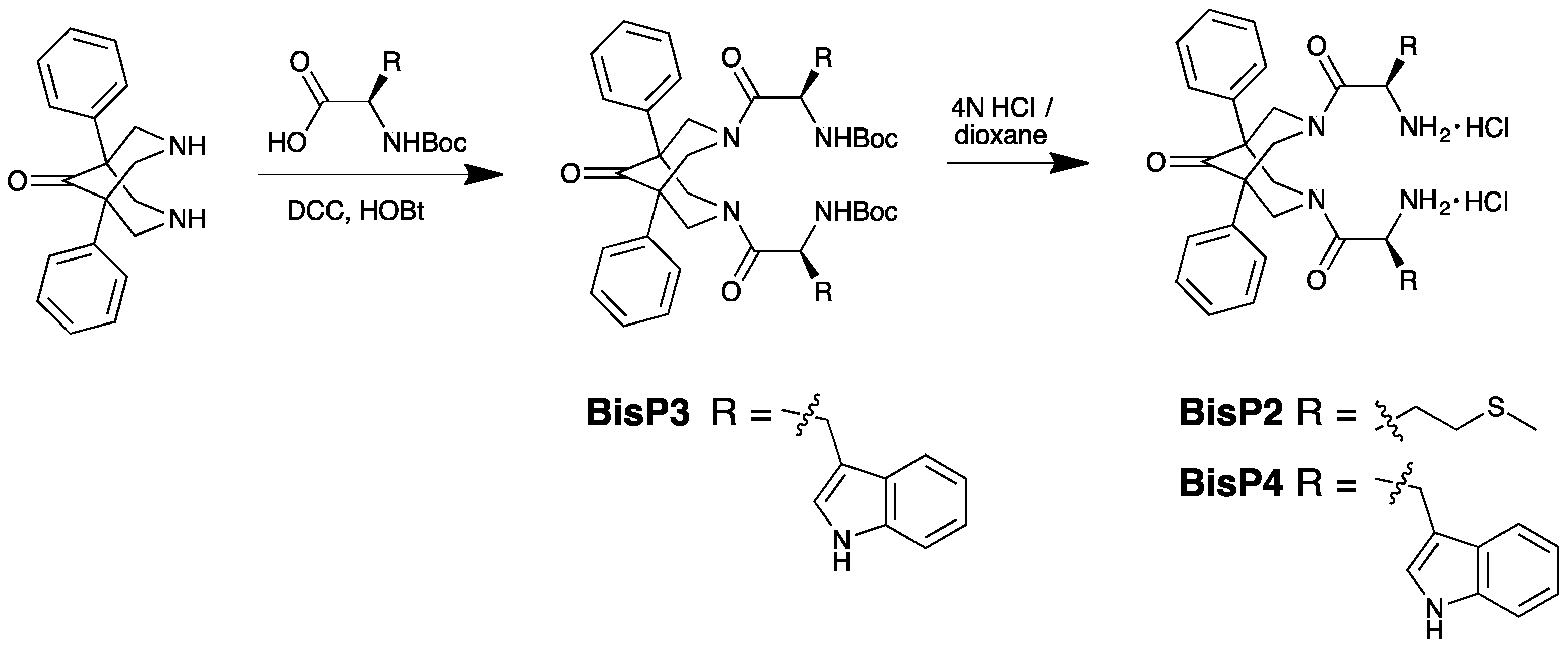
2.1. Synthesis of BisP1-BisP4
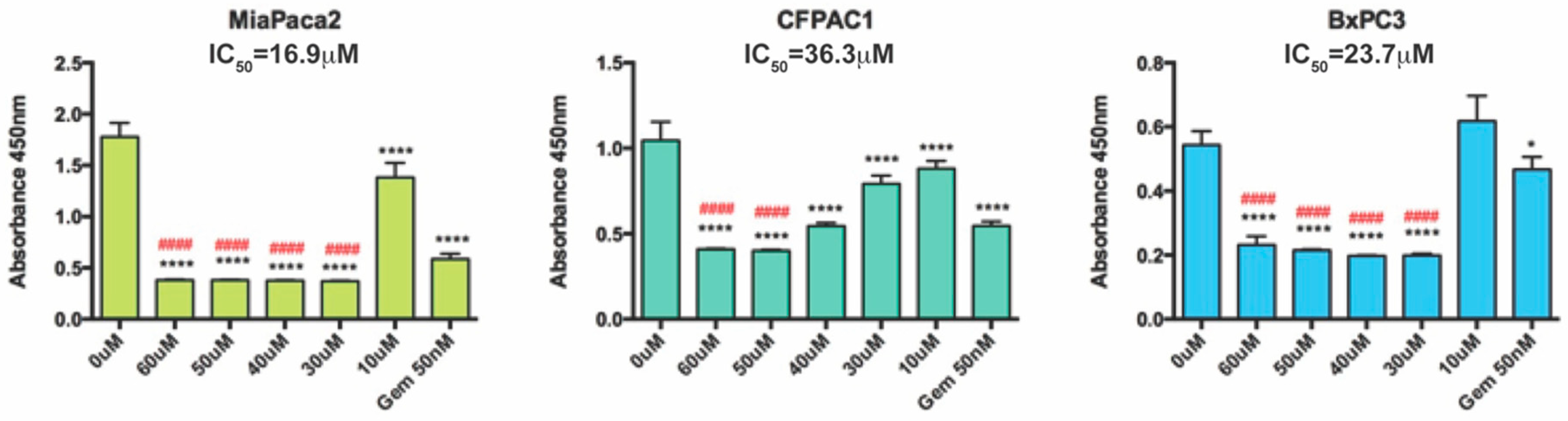
2.2. Cytotoxic Effect of Bispidinone Treatment
2.3. BisP4 Induces Apoptosis in Pancreatic Cancer Cells
2.4. Cell Death Analysis by Flow Cytometry
3. Discussion
4. Materials and Methods
4.1. Chemistry General Procedures
4.2. Preparation of Bispidinone Compounds
4.3. General Cell Procedures
4.3.1. Cell Culture
4.3.2. Cell Viability Assays
4.3.3. Measurement of Apoptotic Cell Morphology
4.3.4. Cell Death Analysis by Fluorescent Staining
4.3.5. Cell Death Analysis by Flow Cytometry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Oberstein, P.E.; Olive, K.P. Pancreatic cancer: Why is it so hard to treat? Ther. Adv. Gastroenterol. 2013, 6, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zhang, Y.; Yu, X.; Yang, J.; LeBrun, D.G.; Chen, C.; Yao, Q.; Li, M. Overcoming drug resistance in pancreatic cancer. Expert Opin. Ther. Targets 2011, 15, 817–828. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New horizons for old drugs and drug leads. J. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Borsodi, A.; Benyhe, S.; Holzgrabe, U.; Márki, Á.; Nachtsheim, C. Structurally novel group of ligands selective for kappa opioid receptors. Regul. Pept. 1994, 54, 27–28. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Somenzi, O.; Picciotto, M.R. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology 2007, 52, 1256–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, E.G.; Mendez-Galvez, C.; Cassels, B.K. Cytisine: A natural product lead for the development of drugs acting at nicotinic acetylcholine receptors. Nat. Prod. Rep. 2012, 29, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Rouden, J.; Lasne, M.C.; Blanchet, J.; Baudoux, J. (−)-Cytisine and derivatives: Synthesis, reactivity, and applications. Chem. Rev. 2014, 114, 712–778. [Google Scholar] [CrossRef]
- Ruenitz, P.C.; Mokler, C.M. Analogues of sparteine. 5. Antiarrhythmic activity of selected N,N′-disubstituted bispidines. J. Med. Chem. 1977, 20, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Galasso, V.; Goto, K.; Miyahara, Y.; Kovac, B.; Klasnic, L. On the structure and spectroscopic properties of bispidine, N,N′-dimethylbispidine and a bis-bispidine macrocycle. Chem. Phys. 2002, 277, 229–240. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Krut’ko, D.P.; Zyk, N.V.; Zefirov, N.S.; Churakov, A.V.; Churakov, A.M.; Howard, J.A. First 1H NMR observation of chair-boat conformers in bispidinone system. Molecular structure of 3,7-diisopropyl-1,5-diphenyl-3,7-diazabicyclo-[3.3.1]nonane-9-one. Mendeleev Commun. 1999, 9, 103–105. [Google Scholar] [CrossRef]
- Tomassoli, I.; Gundisch, D. Bispidine as a Privileged Scaffold. Curr. Top. Med. Chem. 2016, 16, 1314–1342. [Google Scholar] [CrossRef] [PubMed]
- Haridas, V.; Sadanandan, S.; Sharma, Y.K.; Chinthalapalli, S.; Shandilya, A. Bispidine as a secondary structure nucleator in peptides. Tetrahedron Lett. 2012, 53, 623–626. [Google Scholar] [CrossRef]
- Haridas, V.; Rajgokul, K.S.; Sandanandan, S.; Agrawal, T.; Sharvani, V.; Gopalakrishna, M.V.S.; Bijesh, M.B.; Kumawat, K.L.; Basu, A.; Medigeshi, G.R. Bispidine-Amino Acid conjugates act as a novel scaffold for the design of antivirals that block Japanese encephalitis virus replication. PLoS Negl. Trop. Dis. 2013, 7, e2005. [Google Scholar] [CrossRef] [PubMed]
- Chiavarelli, S.; Settimj, G.; Alves, H. Synthesis of 1,5-diphenylbispidin-9-one and 1,5-diphenylbispidin-9-ol series. Gazz. Chim. Ital. 1957, 87, 109–119. [Google Scholar]
- Chiavarelli, S.; Toeffler, F.; Landi Vittory, R.; Mazzeo, P. Synthesis of 1,5-diphenylbispidin-9-ones. VII. 1,5-difenil-3,7-dialkylbispidinones. Gazz. Chim. Ital. 1964, 94, 1021–1027. [Google Scholar]
- Jeyaraman, R.; Avila, S. Chemistry of 3-azabicyclo[3.3.1]nonanes. Chem. Rev. 1981, 81, 149–174. [Google Scholar] [CrossRef]
- Settimj, G.; Landi-Vittory, R.; Delle Monache, F.; Chiavarelli, S. Synthesis of 1,5-diphenylbispidin-9-one and -9-ol derivatives. X. Nitration of dibenzylketone and synthesis of some 3,5-bis(nitrophenyl)-4-piperidones and 1,5-bis(nitrophenyl)-9-bispidinones. Gazz. Chim. Ital. 1966, 96, 311–324. [Google Scholar]
- Juran, S.; Walther, M.; Stephan, H.; Bergmann, R.; Steinbach, J.; Kraus, W.; Emmerling, F.; Comba, P. Hexadentate bispidine derivatives as versatile bifunctional chelate agents for copper(II) radioisotopes. Bioconj. Chem. 2009, 20, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Parthiban, P.; Hwang, J.; Zhang, X.; Jeong, H.; Park, D.H.; Kim, D.K. Effect of a bispidinone analog on mitochondria mediated apoptosis in HeLa cells. Int. J. Oncol. 2014, 44, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhang, X.; Jeong, H.; Shin, Y.M.; Park, D.H.; You, S.; Kim, D. A novel bispidinone analog induces S-phase cell cycle arrest and apoptosis in HeLa human cervical carcinoma cells. Oncol. Rep. 2015, 33, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Black, D.S.C.; Deacon, G.B.; Rose, M. Synthesis and metal complexes of symmetrically N-substituted bispidinones. Tetrahedron 1995, 51, 2055–2076. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2010, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J. Natl. Cancer Inst. 2015, 107, dju413. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Lee, Y.; Kim, R. Harnessing gemcitabine metabolism: A step towards personalized medicine for pancreatic cancer. Ther. Adv. Med. Oncol. 2012, 4, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shendage, D.; Frohlich, R.; Haufe, G. Highly efficient stereoconservative amidation and deamidation of α-amino acids. Org. Lett. 2004, 6, 3675–3678. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunelle, J.K.; Zhang, B. Apoptosis assays for quantifying the bioactivity of anticancer drug products. Drug Resist. Updat. 2010, 13, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Portugal, J.; Bataller, M.; Mansilla, S. Cell death pathways in response to antitumo therapy. Tumori 2009, 95, 409–421. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors conditionally upon request. |



| Bispidinones | Significantly Reduced Cell Viability at ≤50 μM | |||
|---|---|---|---|---|
| MiaPaCa-2 | CFPAC1 | BxPC3 | ||
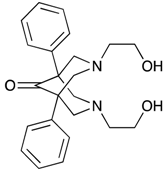 | 1,5-Diphenyl-3,7-bis(2-hydroxyethyl)-3,7-diazabicyclo[3.3.1]nonan-9-one (BisP1) | No | No | No |
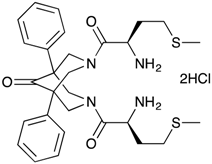 | 3,7-Bis-(2-(S)-amino-4-methylsulfanylbutyryl)-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one dihydrochloride (BisP2) | No | No | No |
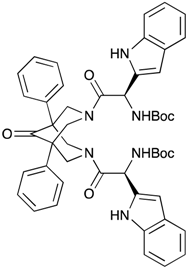 | [2-{7-[2-(S)-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)-propionyl]-9-oxo-1,5-diphenyl-3,7-diazabicyclo[3.3.1]non-3-yl}-1-(S)-(1H-indol-3-ylmethyl)-2-oxoethyl]-carbamic acid tert-butyl ester (BisP3) | ND | ND | ND |
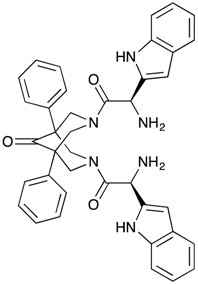 | 3,7-Bis-[2-(S)-amino-3-(1H-indol-3-yl)-propionyl]-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one dihydrochloride (BisP4) | Yes a | Yes a | Yes b |
| MiaPaca-2 Treatment | % Live | % Apoptotic | % Apoptotic/ Dead | % Dead | % Total Apoptotic |
|---|---|---|---|---|---|
| Control | 79.8 ± 0.7 | 5.8 ± 1.5 | 10.9 ± 2.0 | 4.0 ± 3.0 | 16.2 ± 3.5 |
| 17.5 µM | 77.8 ± 5.5 | 4.7 ± 2.3 | 15.2 ± 3.7 | 2.3 ± 0.3 | 19.9 ± 5.9 |
| 26 µM | 35.8 ± 4.1 | 1.2 ± 0.2 | 57.2 ± 4.0 | 5.8 ± 0.4 | 59.5 ± 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predebon, M.J.; Bond, D.R.; Brzozowski, J.; Jankowski, H.; Deane, F.; Tarleton, M.; Shaw, A.A.; McCluskey, A.; Bowyer, M.C.; Weidenhofer, J.; et al. The Bispidinone Derivative 3,7-Bis-[2-(S)-amino-3-(1H-indol-3-yl)-propionyl]-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one Dihydrochloride Induces an Apoptosis-Mediated Cytotoxic Effect on Pancreatic Cancer Cells In Vitro. Molecules 2019, 24, 524. https://doi.org/10.3390/molecules24030524
Predebon MJ, Bond DR, Brzozowski J, Jankowski H, Deane F, Tarleton M, Shaw AA, McCluskey A, Bowyer MC, Weidenhofer J, et al. The Bispidinone Derivative 3,7-Bis-[2-(S)-amino-3-(1H-indol-3-yl)-propionyl]-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one Dihydrochloride Induces an Apoptosis-Mediated Cytotoxic Effect on Pancreatic Cancer Cells In Vitro. Molecules. 2019; 24(3):524. https://doi.org/10.3390/molecules24030524
Chicago/Turabian StylePredebon, Melanie J., Danielle R. Bond, Joshua Brzozowski, Helen Jankowski, Fiona Deane, Mark Tarleton, Aron A. Shaw, Adam McCluskey, Michael C. Bowyer, Judith Weidenhofer, and et al. 2019. "The Bispidinone Derivative 3,7-Bis-[2-(S)-amino-3-(1H-indol-3-yl)-propionyl]-1,5-diphenyl-3,7-diazabicyclo[3.3.1]nonan-9-one Dihydrochloride Induces an Apoptosis-Mediated Cytotoxic Effect on Pancreatic Cancer Cells In Vitro" Molecules 24, no. 3: 524. https://doi.org/10.3390/molecules24030524





