Flow Cytometry Analysis of Antibacterial Effects of Universal Dentin Bonding Agents on Streptococcus mutans
Abstract
:1. Introduction
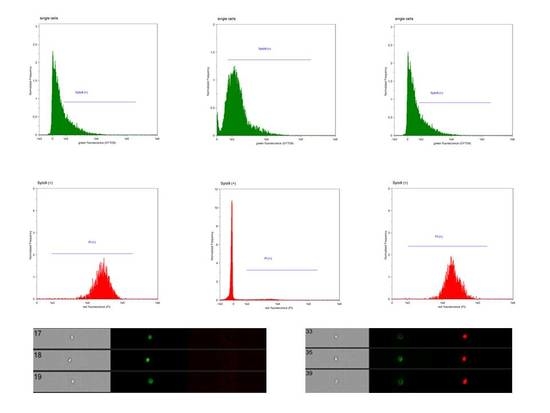
2. Results
3. Discussion
4. Materials and Methods
4.1. Eluate Preparation
4.2. Microbank System
4.3. Bacteria Suspension Preparation
4.4. Bacteria Incubation
4.5. Fluorescent Staining Procedure
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settembrini, L.; Boylan, R.; Strassler, H.; Scherer, W. A comparison of antimicrobial activity of etchants used for a total etch technique. Oper. Dent. 1997, 22, 84–88. [Google Scholar] [PubMed]
- Lukomska-Szymanska, M.; Sokolowski, J.; Lapinska, B. Chlorhexidine—Mechanism of action and its application to dentistry. J. Stomatol. 2017, 70, 405–417. [Google Scholar]
- Lukomska-Szymanska, M.; Zarzycka, B.; Grzegorczyk, J.; Poltorak, K.; Sokolowski, J.; Lapinska, B. Streptococcus mutans and Enterococcus faecalis as crucial pathogens of oral cavity. Dent. Forum 2016, 44, 47–52. [Google Scholar]
- Lapinska, B.; Klimek, L.; Sokolowski, J.; Lukomska-Szymanska, M. Dentine Surface Morphology after Chlorhexidine Application—SEM Study. Polymers 2018, 10, 905. [Google Scholar] [CrossRef]
- Carrilho, M.R.; Carvalho, R.M.; Sousa, E.N.; Nicolau, J.; Breschi, L.; Mazzoni, A.; Tjäderhane, L.; Tay, F.R.; Agee, K.; Pashley, D.H. Substantivity of chlorhexidine to human dentin. Dent. Mater. 2010, 26, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocco, A.R.; De Oliveira Da Rosa, W.L.; Da Silva, A.F.; Lund, R.G.; Piva, E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent. Mater. 2015, 31, 1345–1362. [Google Scholar] [CrossRef]
- Penmetsa, R.K.R.; Sri Rekha, A.; Poppuri, K.C.; Sai Prashanth, P.; Garapati, S. An invitro evaluation of antibacterial properties of self etching dental adhesive systems. J. Clin. Diagn. Res. 2014, 8, ZC01-5. [Google Scholar] [CrossRef]
- Amin, S.; Shetty, H.K.; Varma, R.K.; Amin, V.; Nair, P.M.S. Comparative evaluation of antibacterial activity of total-etch and self-etch adhesive systems: An ex vitro study. J. Conserv. Dent. 2014, 17, 266–270. [Google Scholar] [CrossRef]
- Ozel, E.; Kolayli, F.; Tuna, E.B.; Er, D. In vitro antibacterial activity of various adhesive materials against oral streptococci. Biotechnol. Biotechnol. Equip. 2016, 30, 121–126. [Google Scholar] [CrossRef]
- Lukomska-Szymanska, M.; Olbert-Sieroszewska, V.; Zurawska-Olszewska, J.; Szczerba, I.; Krzeminski, Z.; Sokolowski, J. Antibacterial Properties of Total-Etch Bonding Systems. Pol. J. Environ. Stud. 2009, 18, 267–273. [Google Scholar]
- Esteves, C.M.; Ota-Tsuzuki, C.; Reis, A.F.; Rodrigues, J.A. Antibacterial Activity of Various Self-etching Adhesive Systems Against Oral Streptococci. Oper. Dent. 2010, 35, 448–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, O.; Matalon, S.; Slutzky, H.; Weiss, E.I. Antibacterial properties of self-etching dental adhesive systems. J. Am. Dent. Assoc. 2007, 138, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.; Mayer, M. Enterococcus faecalis in Oral Infections. JBR J. Interdiscip. Med. Dent. Sci. 2014, 3, 160. [Google Scholar]
- Marsh, P.; Martin, M. Mikrobiologia Jamy Ustnej; PWN: Warsaw, Poland, 1994. [Google Scholar]
- Hahnel, S.; Leyer, A.; Rosentritt, M.; Handel, G.; Bürgers, R.; Burgers, R.; Bürgers, R. Surface properties and in vitro Streptococcus mutans adhesion to self-etching adhesives. J. Adhes. Dent. 2009, 11, 263–269. [Google Scholar] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.; Makishi, P.; Almeida Ayres, A.P.; Moreira Vermelho, P.; Marin Fronza, B.; Nikaido, T.; Tagami, J. Self-Etch Adhesive Systems: A Literature Review. Braz. Dent. J. 2015, 26, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lukomska-Szymanska, M.; Sokolowski, J.; Lapinska, B. Current views on adhesive bonding systems. J. Stomatol. 2017, 70, 384–393. [Google Scholar]
- Sato, T.; Takagaki, T.; Matsui, N.; Hamba, H.; Sadr, A.; Nikaido, T.; Tagami, J. Morphological Evaluation of the Adhesive/Enamel interfaces of Two-step Self-etching Adhesives and Multimode One-bottle Self-etching Adhesives. J. Adhes. Dent. 2016, 18, 1–5. [Google Scholar]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; Van Meerbeek, B. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Paradella, T.C.; Koga-Ito, C.Y.; Jorge, A.O.C. In vitro antibacterial activity of adhesive systems on Streptococcus mutans. J. Adhes. Dent. 2009, 11, 95–99. [Google Scholar] [PubMed]
- Imazato, S.; Ehara, A.; Torii, M.; Ebisu, S. Antibacterial activity of dentine primer containing MDPB after curing. J. Dent. 1998, 26, 267–271. [Google Scholar] [CrossRef]
- Imazato, S.; Russell, R.R.; McCabe, J.F. Antibacterial activity of MDPB polymer incorporated in dental resin. J. Dent. 1995, 23, 177–181. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y.; et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of streptococcus mutans biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Imai, T.; Ebisu, S. Antibacterial activity of proprietary self-etching primers. Am. J. Dent. 1998, 11, 106–108. [Google Scholar] [PubMed]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Luddin, N.; Ahmed, H.M. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J. Conserv. Dent. 2013, 16, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Vital, M.; Hülshoff, I.; Weilenmann, H.U.; Egli, T.; Hammes, F. Rapid, cultivation-independent assessment of microbial viability in drinking water. Water Res. 2008, 42, 4010–4018. [Google Scholar] [CrossRef]
- Hewitt, C.J.; Nebe-von-Caron, G. The Application of multi-parameter flow cytometry to monitor individual microbial cell physiological state. Adv. Biochem. Eng. Biotechnol. 2004, 89, 197–223. [Google Scholar]
- Brunzel, S.; Yang, B.; Wolfart, S.; Kern, M. Tensile bond strength of a so-called self-adhesive luting resin cement to dentin. J. Adhes. Dent. 2010, 12, 143–150. [Google Scholar]
- Leif, R.C. Book Review: Practical flow cytometry, 3rd Edition, by Howard M. Shapiro, M.D., Wiley-Liss, Inc., New York, 1995, 542 pages. Cytometry Part A 1995, 19, 376. [Google Scholar] [CrossRef]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Hammes, F.; Canette, A.; Bouchez, T.; Briandet, R. Fluorescence-based tools for single-cell approaches in food microbiology. Int. J. Food Microbiol. 2015, 213, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Buysschaert, B.; Byloos, B.; Leys, N.; Van Houdt, R.; Boon, N. Reevaluating multicolor flow cytometry to assess microbial viability. Appl. Microbiol. Biotechnol. 2016, 100, 9037–9051. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, L.F.; Fuller, K.A.; Erber, W.N. Applications of imaging flow cytometry in the diagnostic assessment of acute leukaemia. Methods 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, T.A.; Madkaikar, M.; Rosenzweig, S.D. Application of Flow Cytometry in the Evaluation of Primary Immunodeficiencies. Indian J. Pediatr. 2016, 83, 444–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukomska-Szymanska, M.; Konieczka, M.; Zarzycka, B.; Lapinska, B.; Grzegorczyk, J.; Sokolowski, J. Antibacterial activity of commercial dentine bonding systems against E. faecalis-flow cytometry study. Materials 2017, 10, 481. [Google Scholar] [CrossRef]
- Lukomska-Szymanska, M.; Sokolowski, J.; Lapinska, B. Degradation of a hybrid layer—Review of literature. J. Stomatol. 2017, 70, 88–94. [Google Scholar]
- Hiraishi, N.; Yiu, C.K.Y.; King, N.M.; Tay, F.R. Effect of 2% chlorhexidine on dentin microtensile bond strengths and nanoleakage of luting cements. J. Dent. 2009, 37, 440–448. [Google Scholar] [CrossRef]
- Shafiei, F.; Memarpour, M. Antibacterial activity in adhesive dentistry: A literature review. Gen. Dent. 2012, 60, e346–e356. [Google Scholar]
- Lukomska-Szymanska, M.; Zarzycka, B.; Grzegorczyk, J.; Sokolowski, K.; Poltorak, K.; Sokolowski, J.; Lapinska, B. Antibacterial Properties of Calcium Fluoride-Based Composite Materials: In Vitro Study. BioMed Res. Int. 2016, 2016, 1048320. [Google Scholar] [PubMed]
- Poggio, C.; Arciola, C.R.; Cepurnykh, S.; Chiesa, M.; Scribante, A.; Selan, L.; Imbriani, M.; Visai, L. In vitro antibacterial activity of different self-etch adhesives. Int. J. Artif. Organs 2012, 35, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chai, Z.G.; Sun, M.N.; Wang, F.; Ma, S.; Zhang, L.; Fang, M.; Chen, J.H. Anti-biofilm effect of dental adhesive with cationic monomer. J. Dent. Res. 2009, 88, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imazato, S.; Ma, S.; Chen, J.H.; Xu, H.H.K. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent. Mater. 2014, 30, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giammanco, G.M.; Cumbo, E.M.G.; Luciani, A.; Gallina, G.; Mammina, C.; Pizzo, G. In vitro evaluation of the antibacterial activity of cured dentin/enamel adhesive incorporating the antimicrobial agent MDPB. New Microbiol. 2009, 32, 385–390. [Google Scholar] [PubMed]
- Imazato, S.; Kuramoto, A.; Takahashi, Y.; Ebisu, S.; Peters, M.C. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent. Mater. 2006, 22, 527–532. [Google Scholar] [CrossRef]
- Li, F.; Chen, J.; Chai, Z.; Zhang, L.; Xiao, Y.; Fang, M.; Ma, S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J. Dent. 2009, 37, 289–296. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Boening, K.W.; Grychowska, N.; Paradowska-Stolarz, A. Clinical Application of Chitosan in Dental Specialities. Mini-Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef]
- Sokolowski, K.; Szynkowska, M.I.; Pawlaczyk, A.; Lukomska-Szymanska, M.; Sokolowski, J. The impact of nanosilver addition on element ions release form light-cured dental composite and compomer into 0.9% NaCl. Acta Biochim. Pol. 2014, 61, 317–323. [Google Scholar]
- Łukomska-Szymańska, M.; Kleczewska, J.; Nowak, J.; Pryliński, M.; Szczesio, A.; Podlewska, M.; Sokołowski, J.; Łapińska, B. Mechanical Properties of Calcium Fluoride-Based Composite Materials. BioMed Res. Int. 2016, 2016, 2752506. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, K.; Kleczewska, J.; Krasowski, M.; Lapinska, B.; Sokolowski, J. The Influence of Amorphous Calcium Phosphate Addition on Mechanical Properties of the Experimental Light-Cured Dental Composite. Dent. Med. Probl. 2016, 53, 34–40. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-H.; Chen, J.-H.; Fang, M.; Xing, X.-D.; Wang, H.; Wang, Y.-J.; Li, F. Antibacterial effects of three experimental quaternary ammonium salt (QAS) monomers on bacteria associated with oral infections. J. Oral Sci. 2008, 50, 323–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Torii, M.; Russell, R.R.B.; McCabe, J.F. Incorporation of Antibacterial Monomer MDPB into Dentin Primer. J. Dent. Res. 1997, 76, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ebi, N.; Tarumi, H.; Russell, R.R.B.; Kaneko, T.; Ebisu, S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials 1999, 20, 899–903. [Google Scholar] [CrossRef]
- de Carvalho, F.G.; Puppin-Rontani, R.M.; de Fúcio, S.B.P.; Negrini, T.; Carlo, H.L.; Garcia-Godoy, F. Analysis by confocal laser scanning microscopy of the MDPB bactericidal effect on S. mutans biofilm CLSM analysis of MDPB bactericidal effect on biofilm. J. Appl. Oral Sci. 2012, 20, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, Y.; Ozalp, M.; Attar, N. Comparison of the antibacterial activity of different self-etching primers and adhesives. J. Contemp. Dent. Pract. 2008, 9, 057–064. [Google Scholar]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef]
- Brambilla, E.; Ionescu, A.; Fadini, L.; Mazzoni, A.; Imazato, S.; Pashley, D.; Breschi, L.; Gagliani, M. Influence of MDPB-containing primer on Streptococcus mutans biofilm formation in simulated Class I restorations. J. Adhes. Dent. 2013, 15, 431–438. [Google Scholar]
- Imazato, S.; Kaneko, T.; Takahashi, Y.; Noiri, Y.; Ebisu, S. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper. Dent. 2004, 29, 369–375. [Google Scholar] [PubMed]
- Chai, Z.; Li, F.; Fang, M.; Wang, Y.; Ma, S.; Xiao, Y.; Huang, L.; Chen, J. The bonding property and cytotoxicity of a dental adhesive incorporating a new antibacterial monomer. J. Oral Rehabil. 2011, 38, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Camilleri, J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review. Dent. Mater. 2015, 31, e89–e99. [Google Scholar] [CrossRef] [PubMed]
- Cehreli, Z.C.; Stephan, A.; Sener, B. Antimicrobial properties of self-etching primer-bonding systems. Oper. Dent. 2003, 28, 143–148. [Google Scholar] [PubMed]
- Imazato, S.; Kuramoto, A.; Kaneko, T.; Ebisu, S.; Russell, R.R.B. Comparison of antibacterial activity of simplified adhesive systems. Am. J. Dent. 2002, 15, 356–360. [Google Scholar] [PubMed]
- Imazato, S.; Imai, T.; Russell, R.R.B.; Torii, M.; Ebisu, S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J. Biomed. Mater. Res. 1998, 39, 511–515. [Google Scholar] [CrossRef]
- Huang, L.; Yu, F.; Sun, X.; Dong, Y.; Lin, P.T.; Yu, H.H.; Xiao, Y.H.; Chai, Z.G.; Xing, X.D.; Chen, J.H. Antibacterial activity of a modified unfilled resin containing a novel polymerizable quaternary ammonium salt MAE-HB. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Daood, U.; Yiu, C.K.Y. Transdentinal cytotoxicity and macrophage phenotype of a novel quaternary ammonium silane cavity disinfectant. Dent. Mater. 2019, 35, 206–216. [Google Scholar] [CrossRef]
- Vaidyanathan, M.; Sheehy, E.C.; Gilbert, S.C.; Beighton, D. Antimicrobial properties of dentine bonding agents determined using in vitro and ex vivo methods. J. Dent. 2009, 37, 514–521. [Google Scholar] [CrossRef]
- Ohmori, K.; Maeda, N.; Kohno, A. Evaluation of antibacterial activity of three dentin primers using an in vitro tooth model. Oper. Dent. 1999, 24, 279–285. [Google Scholar]
- Özer, F.; Karakaya, Ş.; Ünlü, N.; Erganiş, O.; Kav, K.; Imazato, S. Comparison of antibacterial activity of two dentin bonding systems using agar well technique and tooth cavity model. J. Dent. 2003, 31, 111–116. [Google Scholar] [CrossRef]
- Türkün, L.S.; Ateş, M.; Türkün, M.; Uzer, E.; Esra, T. Antibacterial Activity of Two Adhesive Systems Using Various Microbiological Methods. J. Adhes. Dent. 2005, 7, 315–320. [Google Scholar] [PubMed]
- Lanza, C.R.M.; De Souza Costa, C.A.; Furlan, M.; Alécio, A.; Hebling, J. Transdentinal diffusion and cytotoxicity of self-etching adhesive systems. Cell Biol. Toxicol. 2009, 25, 533–543. [Google Scholar] [CrossRef]
- Leite, M.L.; Costa, C.A.; Duarte, R.M.; de Andrade, A.K.M.; Soares, D.G. Bond strength and cytotoxicity of a universal adhesive according to the hybridization strategies to dentin. Braz. Dent. J. 2018, 29, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.C.C.M.; Oliveira, D.C.; Raele, R.A.; Ribas, K.H.S.; Montes, M.A.J.R.; De Castro, C.M.M.B. Cytotoxicity of current adhesive systems: In vitro testing on cell cultures of primary murine macrophages. Dent. Mater. 2011, 27, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P.D.; Botha, F.S.; de Wet, F.A. Antibacterial properties of five bonding agents. SADJ 2008, 63, 448–451. [Google Scholar] [PubMed]
- Asar, N.V.; Korkmaz, T.; Gül, E.B. The effect of wollastonite incorporation on the linear firing shrinkage and flexural strength of dental aluminous core ceramics: A preliminary study. Mater. Des. 2010, 31, 2540–2545. [Google Scholar] [CrossRef]
- Arora, R.; Rao, M.H. Comparative evaluation of the antibacterial effects of four dentine bonding systems: An in vitro study. J. Conserv. Dent. 2013, 16, 466–470. [Google Scholar] [CrossRef]
- Lobo, M.M.; Gonçalves, R.B.; Pimenta, L.A.F.; Bedran-Russo, A.K.B.; Pereira, P.N.R. In vitro evaluation of caries inhibition promoted by self-etching adhesive systems containing antibacterial agents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 122–127. [Google Scholar] [CrossRef]
- Sampath, P.; Hegde, M.; Hegde, P.; Shetty, V. Assessment of antibacterial activity of self-etching dental adhesive systems: An in vitro study. J. Conserv. Dent. 2008, 11, 150–153. [Google Scholar] [CrossRef]
- Loguercio, A.; Luque-Martinez, I.; Muñoz, M.; Szesz, A.; Cuadros-Sánchez, J.; Reis, A. A Comprehensive Laboratory Screening of Three-Step Etch-and-Rinse Adhesives. Oper. Dent. 2014, 39, 652–662. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Van Meerbeek, B.; Yoshida, Y.; Inoue, S.; Vargas, M.; Suzuki, K.; Lambrechts, P.; Vanherle, G. Four-year Water Degradation of Total-etch Adhesives Bonded to Dentin. J. Dent. Res. 2003, 82, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Lukomska-Szymanska, M.; Zarzycka, B.; Zurawska-Olszewska, J.; Olbert-Sieroszewska, V.; Sokolowski, K.; Sokolowski, J. Antibacterial activity of eight dentine bonding systems. IOSR J. Dent. Med. Sci. 2014, 13, 48–54. [Google Scholar] [CrossRef]
- Polydorou, O.; Rogatti, P.; Bolek, R.; Wolkewitz, M.; Kümmerer, K.; Hellwig, E. Elution of monomers from three different bonding systems and their antibacterial effect. Odontology 2013, 101, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Başeren, M.; Yazici, A.R.; Ozalp, M.; Dayangaç, B. Antibacterial activity of different generation dentin-bonding systems. Quintessence Int. 2005, 36, 339–344. [Google Scholar] [PubMed]
- Einhorn, M.; DuVall, N.; Wajdowicz, M.; Brewster, J.; Roberts, H. Preparation Ferrule Design Effect on Endocrown Failure Resistance. J. Prosthodont. 2017, 12, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.; Younes, F.; Lehmann, F.; Kern, M. Tensile Bond Strength of So-called Universal Primers and Universal Multimode Adhesives to Zirconia and Lithium Disilicate Ceramics. J. Adhes. Dent. 2017, 19, 221–228. [Google Scholar]
- Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Photopolymerization of phosphoric acid ester-based self-etch dental adhesives. Dent. Mater. J. 2013, 32, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Ham, Y.; Kim, T.J. Inhibitory activity of monoacylglycerols on biofilm formation in Aeromonas hydrophila, Streptococcus mutans, Xanthomonas oryzae, and Yersinia enterocolitica. Springerplus 2016, 5, 1526. [Google Scholar] [CrossRef]
- Miletic, V.; Santini, A.; Trkulja, I. Quantification of monomer elution and carbon-carbon double bonds in dental adhesive systems using HPLC and micro-Raman spectroscopy. J. Dent. 2009, 37, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Olivier, A.; Grobler, S.R.; Osman, Y. Cytotoxicity of seven recent dentine bonding agents on mouse 3T3 fibroblast cells. Open J. Stomatol. 2012, 2, 244–250. [Google Scholar] [CrossRef]
- Barbosa, M.O.; de Carvalho, R.V.; Demarco, F.F.; Ogliari, F.A.; Zanchi, C.H.; Piva, E.; da Silva, A.F. Experimental self-etching HEMA-free adhesive systems: Cytotoxicity and degree of conversion. J. Mater. Sci. Mater. Med. 2015, 26, 5370. [Google Scholar] [CrossRef] [PubMed]
- Kasacka, I.; Lapinska, J. Salivary cells in patients with dental amalgam and composite resin material restorations—A morphological investigation. Pol. J. Environ. Stud. 2010, 19, 1223–1227. [Google Scholar]
- de Albuquerque, E.G.; Santana, F.W.K.; Calazans, F.S.; Poubel, L.A.; Marins, S.S.; Matos, T.D.P.; Hanzen, T.A.; Barceleiro, M.D.O.; Loguercio, A.D. A New Universal Simplified Adhesive: 6-Month Randomized multi-center clinical trial. Rev. Bras. Odontol. 2017, 74, 251. [Google Scholar] [CrossRef] [Green Version]
- Mena-Serrano, A.; Kose, C.; De Paula, E.A.; Tay, L.Y.; Reis, A.; Loguercio, A.D.; Perdigão, J. A new universal simplified adhesive: 6-month clinical evaluation. J. Esthet. Restor. Dent. 2013, 25, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; De Paula, E.A.; Hass, V.; Luque-Martinez, I.; Reis, A.; Perdigão, J. A new universal simplified adhesive: 36-Month randomized double-blind clinical trial. J. Dent. 2015, 43, 1083–1092. [Google Scholar] [CrossRef]
- Ruschel, V.; Shibata, S.; Stolf, S.; Chung, Y.; Baratieri, L.; Heymann, H.; Walter, R. Eighteen-month Clinical Study of Universal Adhesives in Noncarious Cervical Lesions. Oper. Dent. 2018, 43, 241–249. [Google Scholar] [CrossRef]
- Çakır, N.N.; Demirbuga, S. The effect of five different universal adhesives on the clinical success of class I restorations: 24-month clinical follow-up. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef]
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| DBS | M | SD | CV | Min.–Max. | |
|---|---|---|---|---|---|
| 1. | Adhese Universal | 99.68 | 0.25 | 0.25% | 99.19–99.87 |
| 2. | Clearfil Universal Bond Quick | 91.13 | 12.91 | 14.17% | 65.62–99.94 |
| 3. | Clearfil SE Bond 2 (Primer) | 84.02 | 15.96 | 18.99% | 56.87–99.33 |
| 4. | Clearfil SE Bond 2 (Bond) | 5.37 | 4.33 | 80.70% | 1.96–13.04 |
| 5. | Clearfil SE Bond 2 (Primer + Bond) | 91.78 | 12.10 | 13.18% | 68.57–99.82 |
| 6. | OptiBond Universal | 94.99 | 7.87 | 8.28% | 79.55–99.84 |
| 7. | OptiBond FL (Primer) | 94.18 | 7.32 | 7.77% | 80.00–99.87 |
| 8. | OptiBond FL (Adhesive) | 4.65 | 3.23 | 69.47% | 1.85–10.66 |
| 9. | OptiBond FL (Primer + Adhesive) | 15.58 | 14.01 | 89.95% | 5.95–42.96 |
| 10. | Prime&Bond Universal | 96.95 | 4.11 | 4.24% | 88.64–99.39 |
| 11. | Universal Bond | 12.68 | 12.40 | 97.77% | 3.37–34.32 |
| 12. | Isopropanol 70% | 97.58 | 3.40 | 3.49% | 88.95–99.82 |
| 13. | NaCl 0.85% | 2.73 | 2.25 | 82.55% | 1.16–8.96 |
| DBS No. | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | |||||||||||||
| 2. | p = 0.088 | ||||||||||||
| 3. | p = 0.002 | p = 0.154 | |||||||||||
| 4. | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||||
| 5. | p = 0.114 | p = 0.897 | p = 0.121 | p < 0.001 | |||||||||
| 6. | p = 0.347 | p = 0.437 | p = 0.029 | p < 0.001 | p = 0.517 | ||||||||
| 7. | p = 0.270 | p = 0.539 | p = 0.043 | p < 0.001 | p = 0.627 | p = 0.870 | |||||||
| 8. | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.042 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||
| 9. | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.042 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.030 | |||||
| 10. | p = 0.583 | p = 0.243 | p = 0.011 | p < 0.001 | p = 0.298 | p = 0.693 | p = 0.577 | p < 0.001 | p < 0.001 | ||||
| 11. | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.143 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.108 | p = 0.560 | p < 0.001 | |||
| 12. | p = 0.631 | p = 0.142 | p = 0.003 | p < 0.001 | p = 0.186 | p = 0.554 | p = 0.437 | p < 0.001 | p < 0.001 | p = 0.886 | p < 0.001 | ||
| 13. | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.539 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.653 | p = 0.004 | p < 0.001 | p = 0.023 | p < 0.001 |
| Name | Manufacturer | Number of Components | Type | Composition | Mode of Etching | ||
|---|---|---|---|---|---|---|---|
| ER 1 | SE 2 | SEE 3 | |||||
| Adhese Universal | Ivoclar Vivadent/Germany | 1 | 1-step | methacrylated phosphoric acid ester (MDP, 3–10%), MCAP methacrylated carboxylic acid polymer, HEMA (10–25%), Bis-GMA (10–25%), D3MA (Decandiol dimethacrylate) (3–10%), 2-dimethylaminoethyl methacrylate (1–2.5%), camphoroquinone (1–2.5%), ethanol (10–25%) | + | + | + |
| Clearfil Universal Bond Quick | Kuraray America/USA | 1 | 1-step | 10-MDP, Bis-GMA (10–25%), HEMA (2.5–10%), ethanol (10–25%) Hydrophilic amide monomers, Colloidal silica, Silane coupling agent, Sodium fluoride, dl-Camphorquinone | + | + | + |
| Clearfil SE Bond 2 (Primer+Bond) | Kuraray America/USA | 2 | 2-step | Primer: 10-MDP, HEMA (20–40%), Hydrophilic aliphatic dimethacrylate, dl-Camphorquinone Bond: 10-MDP, Bis-GMA (25–45%), HEMA (20–40%), Hydrophobic aliphatic dimethacrylate, dl-Camphorquinone, Silanated colloidal silica | + | ||
| OptiBond Universal | Kerr/USA | 1 | 1-step | Acetone (30–60%), HEMA (5–10%), Glycerol Dimethacrylate (1–5%), ethanol (5–10%) | + | + | + |
| OptiBond FL (Primer+Adhesive) | Kerr/USA | 2 | 3-step | Primer: HEMA (10–30%), ethanol (10–30%), 4-MET (10–30%), glycerol phosphate dimethacrylate (5–10%) Adhesive: HEMA (10–30%), ytterbium trifluoride, 3-trimethoxysilylpropyl methacrylate (5–10%), 2-hydroxy-1,3-propanediyl bismethacrylate (5–10%), alkali fluorosilicates(Na) (1–5%) | + | ||
| Prime&Bond Universal | Dentsply/UK | 1 | 1-step | Phosphoric acid modified acrylate resin, Multifunctional acrylate, Bifunctional acrylate, Acidic acrylate, Isopropanol, Water, Initiator | + | + | + |
| Universal Bond | Tokuyama/Japan | 2 | 1-step | Phosphoric acid monomer (1–5%), Bisphenol A, Bis-GMA, TEGDMA, HEMA (10–30%), MTU-6 (thiouracil monomer), Silane coupling agent, Peroxide, Borate catalyst, Acetone, Isopropanol | + | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapinska, B.; Konieczka, M.; Zarzycka, B.; Sokolowski, K.; Grzegorczyk, J.; Lukomska-Szymanska, M. Flow Cytometry Analysis of Antibacterial Effects of Universal Dentin Bonding Agents on Streptococcus mutans. Molecules 2019, 24, 532. https://doi.org/10.3390/molecules24030532
Lapinska B, Konieczka M, Zarzycka B, Sokolowski K, Grzegorczyk J, Lukomska-Szymanska M. Flow Cytometry Analysis of Antibacterial Effects of Universal Dentin Bonding Agents on Streptococcus mutans. Molecules. 2019; 24(3):532. https://doi.org/10.3390/molecules24030532
Chicago/Turabian StyleLapinska, Barbara, Magdalena Konieczka, Beata Zarzycka, Krzysztof Sokolowski, Janina Grzegorczyk, and Monika Lukomska-Szymanska. 2019. "Flow Cytometry Analysis of Antibacterial Effects of Universal Dentin Bonding Agents on Streptococcus mutans" Molecules 24, no. 3: 532. https://doi.org/10.3390/molecules24030532