Olive Leaf Addition Increases Olive Oil Nutraceutical Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quality Parameters
2.2. Free Acidity
2.3. Peroxide Value
2.4. Chlorophylls and Carotenoids
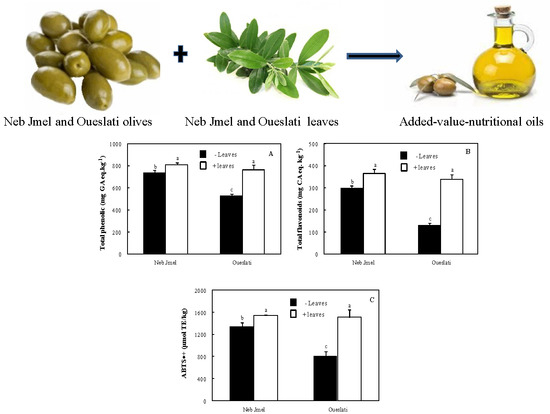
2.5. Total Phenolics
2.6. Total Flavonoids
2.7. ABTS•+ Scavenging Activity
2.8. Phenolic Compounds
2.9. Tocopherols
3. Materials and Methods
3.1. Olive Leaves and Fruit Sampling
3.2. Methods
3.2.1. Quality Parameters
3.2.2. Pigment Concentration
Oil Pigment Determination
Leaf Pigment Determination
3.3. Extraction of Phenolic Compounds
3.3.1. Fresh Leaves
3.3.2. Olive Oils
3.4. Total Polyphenol and Flavonoid Concentrations
3.5. Free-Radical Scavenging Ability
3.6. HPLC Analysis of Phenolic Compounds
3.7. Extraction and Detection of Tocopherols (Vitamin E)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boudhrioua, N.; Bahloul, N.; Ben Slimen, I.; Kechaou, N. Comparison on the total phenol concentrations and the color of fresh and infrared dried olive leaves. Ind. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Andrich, L.; Silvestri, S.; Andrich, G. A kinetic method to evaluate the effect of environmental variability on the quality of an extra virgin olive oil. Agrochimica 2014, 58, 35–50. [Google Scholar]
- Bouaziz, M.; Chamkha, M.; Sayadi, S. Comparative study on phenolic concentration and antioxidant activity during maturation of the olive cultivar Chemlali from Tunisia. J. Agric. Food Chem. 2004, 52, 5476–5481. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L.; Yaqoob, P. Nutritional and health aspects of olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 685–697. [Google Scholar] [CrossRef]
- Turner, R.; Etiene, N.; Garcia-Alonso, M.; de Pascual-Teresa, S.; Minihane, A.M.; Weinberg, P.D.; Rimbach, G. Antioxidant and anti-atherogenic activities of olive oil phenolics. Int. J. Vitaminol. Nutr. Res. 2010, 75, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.G.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Zinnai, A.; Venturi, F.; Quartacci, M.F.; Sanmartin, C.; Favati, F.; Andrich, G. Solid carbon dioxide to promote the extraction of extra-virgin olive oil. Grasas y Aceites 2016, 67, e121. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of Phenol-Enriched Olive Oil with Phenolic Compounds Extracted from Wastewater Produced by Physical Refining. Nutrients 2017, 9, 916. [Google Scholar] [CrossRef]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf 2-dependent gene expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef]
- Gill, C.I.R.; Boyd, A.; McDermott, E.; McCann, M.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; McGlynn, H.; et al. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Guidotti, V.; Bucciantina, M.; Parri, M.; Nediani, C.; Cerbai, E.; Stefani, M.; Berti, A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010, 21, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Muller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Beltran, G.; Aguilera, M.P.; Del Rio, C.; Sanchez, S.; Martinez, L. Influence of fruit ripening process on the natural antioxidant concentration of Hojiblanca virgin olive oils. Food Chem. 2005, 89, 207–215. [Google Scholar] [CrossRef]
- Gambacorta, G.; Faccia, M.; Previtali, M.A.; Pati, S.; La Notte, E.; Bioano, A. Effects of olive maturation and stoning on quality indices and antioxidant concentration of extra virgin oils (cv. Coratina) during storage. J. Food Sci. 2010, 75, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; Esti, M.; Notte, E. Evaluation of phenolic compounds in virgin olive oil during storage. J. Am. Oil Chem. Soc. 1997, 74, 1259–1264. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Teixeira, H.; Bento, A.; Pereira, J.A. Effect of Olive Leaves Addition during the Extraction Process of Overmature Fruits on Olive Oil Quality. Food Bioprocess Technol. 2013, 6, 509–521. [Google Scholar] [CrossRef]
- Bouaziz, M.; Fki, I.; Jemai, H.; Ayadi, M.; Sayadi, S. Effect of storage on refined and husk olive oils composition: Stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem. 2008, 108, 253–262. [Google Scholar] [CrossRef]
- Bubonja-Sonje, M.; Giacometti, J.; Abram, M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011, 127, 1821–1827. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Litina, D.H.; Moustakas, A.; Kiritsakis, A. Composition and Antioxidant Activity of Olive Leaf Extracts from Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Correia, R.; Félix, S.; Ferreira, P.; Gordon, M.H. Effects of enrichment of refined olive oil with phenolic compounds from olive leaves. J. Agric. Food Chem. 2007, 55, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
- IOC. Norme Commercial Applicable Aux Huiles D’olive Et Aux Huiles De Grignon D’olive; COI/NCn°3 Rev.4; Conseil Olèicole International: Madrid, Spain, 2009. [Google Scholar]
- Gutierrez, F.; Varona, I.; Albi, M.A. Relation of acidity and sensory quality with sterol concentration of olive oil from stored fruit. J. Agric. Food Chem. 2000, 48, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, A.; Gargouri, B.; Flamini, G.; Bouaziz, M. Effect of Agricultural Sites on Differentiation between Chemlali and Neb Jmel Olive Oils. J. Oleo Sci. 2015, 64, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouni, Y.; Flamini, G.; Issaoui, M.; Ben Youssef, N.; Luigi, C.P.; Hammami, M.; Daoud, D.; Mokhta, Z. Volatile compounds and compositional quality of virgin olive oil from Oueslati variety: Influence of geographical origin. Food Chem. 2011, 124, 1770–1776. [Google Scholar]
- Martinéz-Suárez, J.M. Recientes estúdios de la almazara experimental del instituto de la grasa. Rivista Italiana delle Sostanze Grasse 1973, 50, 325–330. [Google Scholar]
- Matthaüs, B.; Guillaume, D.; Gharby, S.; Haddad, A.; Harhar, H.; Charrouf, Z. Effect of processing on the quality of edible argan oil. Food Chem. 2010, 120, 426–432. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Haddad, A.; Matthäus, B.; Charrouf, Z. Oxidative Stability of Edible Argan Oil: A TwoYear Period Study. LWT Food Sci. Technol. 2011, 44, 1–8. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Perdiguero, S.; Gutiérrez, R.; Olías, J.M. Evaluation of the bitter taste in virgin olive oil. J. Am. Oil Chem. Soc. 1992, 69, 394–395. [Google Scholar] [CrossRef]
- Sgherri, C.; Perez-Lopez, U.; Pinzino, C. Antioxidant properties of food products containing lycopene are increased by the presence of chlorophyll. In Lycopene: Food Sources, Potential Role in Human Health and Antioxidant Effects; Baily, J.R., Ed.; Nova Publisher: Hauppauge, NY, USA, 2015; pp. 39–89. ISBN 978-1-63117-927-3. [Google Scholar]
- Abaza, L.; Taamalli, W.; Ben Temime, S.; Daoud, D.; Gutierrez, F.; Zarrouk, M. Natural antioxidant composition as correlated to stability of some Tunisian virgin olive oils. Rivista Italiano delle Sostanze Gresse 2005, 132, 12–18. [Google Scholar]
- Giovacchino, L.D.; Angerosa, F.; Giacinto, L.D. Effect of mixing leaves with olives on organoleptic quality of oil obtained by centrifugation. J. Am. Oil Chem. Soc. 1996, 73, 371–374. [Google Scholar] [CrossRef]
- Sevim, D.; Tuncay, O. Effect of Olive Leaves Addition before Extraction of Turkish Olive Cultivars on Olive Oil Minor Components and Antioxidant Activity. Open Access Sci. Rep. 2013, 2, 2–8. [Google Scholar]
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar] [PubMed]
- Crozier, A.; Ashihara, H. Plant Secondary Metabolites and the Human Diet; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Ebrahimi, N.; Khanikia, G.J.; Keshavarz, S.A.; Shariatifar, N.; Nabizadeh, R.; Sadighara, P. Spectrophotometric method for quantification of flavonoids in olive oil supplied from Tehran market of Iran. J. Food Saf. Hyg. 2015, 1, 21–24. [Google Scholar]
- Gorinstein, S.; Martin-Belloso, O.; Katrich, E.; Lojek, A.; Cíz, M.; Gligelmo-Miguel, N.; Haruenkit, R.; Park, Y.S.; Jung, S.T.; Trakhtenberg, S. Comparison of the concentrations of the main biochemical compounds and the antioxidant activity of some Spanish olive oils as determined by four different radical scavenging tests. J. Nutr. Biochem. 2003, 14, 154–159. [Google Scholar] [CrossRef]
- Samaniego, S.C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ben Salah, M.; Abdelmelek, H.; Abderraba, M. Study of Phenolic Composition and Biological Activities Assessment of Olive Leaves from different Varieties Grown in Tunisia. J. Med. Chem. 2012, 2, 107–111. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The Role of Vitamin E in Preventing and Treating Osteoarthritis—A Review of the Current Evidence. Front. Pharmacol. 2018, 9, 946. [Google Scholar] [CrossRef]
- Sedef, N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar]
- Cortesi, N.; Rovellini, P.; Fusari, P. Dosaggio dei biofenoli degli oli vergini di oliva: Idrossitirosolo, tirosolo, agliconi secoiridoidi, acidi secoridoidi, lignani e flavonoidi. Rivista Italiana delle Sostanze Grasse 2002, 79, 145–150. [Google Scholar]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- Khoufi, S.; Aloui, F.; Sayadi, S. Extraction of antioxidants from olive mill wastewater and electro-coagulation of exhausted fraction to reduce its toxicity on anaerobic digestion. J. Hazard. Mater. 2008, 151, 531–539. [Google Scholar] [CrossRef] [PubMed]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Azaizeh, H.; Halahlih, F.; Najami, N.; Brunner, D.; Faulstich, M.; Tafesh, A. Antioxidant activity of phenolic fractions in olive mill wastewater. Food Chem. 2012, 134, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S.; Kol, O. Quantitative determination of phenolic compounds using LC-DAD-ESI-MS/MS in cv. Ayvalik olive oils as affected by harvest time. J. Food Meas. Charact. 2017, 11, 226–235. [Google Scholar] [CrossRef]
- Lucas, A.; Martinez, E.; Rincón, J.; Blanco, M.A.; Garcia, I. Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. J. Supercrit. Fluids 2002, 22, 221–228. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Diaz, T.; Sanchez, J.; De Miguel, C.; Martin-Vertedor, D. Total phenolic compounds and tocopherols profiles of seven olive oil varieties grown in the south-west of Spain. J. Oleo Sci. 2014, 63, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.S.; Fernandes, J.O.; Oliveira, M.B. Quantification of free and esterified sterols in Portuguese olive oils by solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2006, 1128, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, O.; Guerfel, M.; Baccouri, B.; Cerretani, L.; Bendini, A.; Lercker, G.; Zarrouk, M.; Daoud Ben Miled, D. Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem. 2008, 109, 743–754. [Google Scholar] [CrossRef]
- Krichene, D.; Taamalli, W.; Daoud, D.; Salvador, M.D.; Fregapane, G.; Zarrouk, M. Phenolic Compounds, Tocopherols and others minor components in virgin olive oils of some Tunisian Varieties. J. Food Biochem. 2007, 31, 179–194. [Google Scholar] [CrossRef]
- Manai-Djebali, H.; Krichène, D.; Ouni, Y.; Gallardo, L.; Sánchez, J.; Osorio, E.; Daoud, D.; Guido, F.; Zarrouk, M. Chemical profiles of five minor olive oil varieties grown in central Tunisia. J. Food Compos. Anal. 2012, 27, 109–119. [Google Scholar] [CrossRef]
- Sgherri, C.; Kadlecova, Z.; Prdossi, A.; Navari-Izzo, F.; Izzo, R. Irrigation with Diluted Seawater Improves the Nutritional Value of Cherry Tomatoes. J. Agric. Food Chem. 2008, 56, 3391–3397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mínguez-Mosquera, M.I.; Rejano-Navarro, L.; Gandul-Rojas, B.; Sánchez-Gómez, A.H.; Garrido-Fernández, J. Color–Pigment Correlation in Virgin Olive Oil. J. Am. Oil Chem. Soc. 1991, 69, 332–336. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Gallina-Toschi, G.T. Effect of Olive Ripening Degree on the Oxidative Stability and Organoleptic Properties of Cv. Nostrana di Brisighella Extra Virgin Olive Oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimentry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azinobis (3-ethylbenzothiazolyne-6-sulfonic acid) radical cation decolorization assay. Methods Enzymol. 1999, 299, 379–389. [Google Scholar]
- Talcott, S.T.; Howard, L.R. Phenolic autoxidation is responsible for color degradation in processed carrot puree. J. Agric. Food Chem. 1999, 47, 2109–2115. [Google Scholar] [CrossRef]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; de la Torre, M.C.; López-Sabater, M.C. Rapid determination of vitamin E in vegetable oils by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2000, 9, 251–254. [Google Scholar] [CrossRef]
- Galatro, A.; Simontacchi, M.; Puntarulo, S. Free radical generation and antioxidant content in chloroplasts from soybean leaves exposed to ultraviolet-B. Physiol. Plant. 2001, 113, 564–570. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Parameter | Neb Jmel | Oueslati | ||
|---|---|---|---|---|
| Oil | Oil + Leaves | Oil | Oil + Leaves | |
| Free acidity (%C18:1) | 0.56 ± 0.05 b | 0.57 ± 0.03 b | 1.00 ± 0.00 a | 0.60 ± 0.01 b |
| Peroxide value (meq O2/kg) | 6.00 ± 0.71 c | 6.00 ± 0.50 c | 34.00 ± 9.53 a | 15.33 ± 3.21 b |
| Chlorophyll (mg/kg) | 8.37 ± 1.32 b | 14.91 ± 2.91 a | 5.33 ± 1.22 c | 16.51 ± 2.95 a |
| Carotenoids (mg/kg) | 2.95 ± 0.13 c | 5.21 ± 0.54 b | 2.76 ± 0.60 c | 7.26 ± 0.61 a |
| Parameter | Neb Jmel | Oueslati |
|---|---|---|
| Chlorophyll (µg/g DW) | 506.08 ± 10.22 b | 829.29 ± 80.06 a |
| Carotenoids (µg/g DW) | 26.90 ± 4.45 b | 44.33 ± 4.38 a |
| Total phenolics (mg GA eq/g DW) | 62.84 ± 1.29 b | 67.91 ± 3.18 a |
| Total flavonoids (mg CA eq/g DW) | 5.85 ± 1.16 b | 13.61 ± 0.81 a |
| ABTS•+ (µmol TE/g DW) | 69.05 ± 3.26 b | 113.84 ± 5.11 a |
| γ (µg/g DW) | tr | tr |
| α (µg/g DW) | 82.37 ± 9.95 a | 10.12 ± 0.84 b |
| Parameter | Neb Jmel | Oueslati |
|---|---|---|
| Secoiridoids | ||
| Oleuropein derivatives | 648.6 ± 92.2 b | 1948.6 ± 96.8 a |
| Phenolic Acids | ||
| Gallic | 1.76 ± 0.65 a | 0.31 ± 0.25 b |
| Protocatechuic | 37.39 ± 8.68 a | 32.39 ± 12.80 a |
| p-Hydroxybenzoic | 0.93 ± 0.05 b | 4.16 ± 0.41 a |
| Chlorogenic | 1.80 ± 0.00 | Tr. |
| Vanillic | 33.84 ± 2.66 a | 12.11 ± 1.36 b |
| Caffeic | 1.01 ± 0.05 a | 1.19 ± 0.61 a |
| Syringic | 2.28 ± 0.55 b | 8.91 ± 2.65 a |
| Vanillin | 2.90 ± 0.11 b | 11.32 ± 3.84 a |
| p-Coumaric | 5.66 ± 0.52 a | 0.87 ± 0.57 b |
| Ferulic | 15.07 ± 0.40 b | 37.18 ± 4.71 a |
| Phenolic Alcohols | ||
| Hydroxytyrosol | 5.75 ± 1.40 b | 24.03 ± 2.43 a |
| Tyrosol | 3.17 ± 0.76 b | 14.46 ± 2.23 a |
| Parameter | Neb Jmel | Oueslati | ||
|---|---|---|---|---|
| Oil | Oil + Leaves | Oil | Oil + Leaves | |
| Secoiridoids | ||||
| Oleuropein derivatives | 194.4 ± 12.1 b | 211.0 ± 1.9 a | 126.4 ± 2.6 c | 187.6 ± 10.5 b |
| Phenolic Acids | ||||
| Gallic | - | - | - | - |
| Protocatechuic | - | - | - | - |
| p-Hydroxybenzoic | - | - | - | - |
| Chlorogenic | - | - | - | - |
| Vanillic | 1.52 ± 0.02 a | 1.44 ± 0.05 a | 0.77 ± 0.14 b | 0.27 ± 0.03 c |
| Caffeic | 0.007 ± 0.003 b | 0.02 ± 0.0001 a | 0.004 ± 0.001 b | 0.015 ± 0.0001 a |
| Syringic | 0.005 ± 0.004 b | Tr. | 0.02 ± 0.01 a | 0.02 ± 0.005 a |
| Vanillin | 0.15 ± 0.0008 a | 0.17 ± 0.01 a | 0.17 ± 0.05 a | 0.11 ± 0.01 a |
| p-Coumaric | 0.11 ± 0.008 b | 0.12 ± 0.0004 b | 0.29 ± 0.01 a | 0.32 ± 0.04 a |
| Ferulic | 0.10 ± 0.005 a | 0.11 ± 0.005 a | 0.11 ± 0.01 a | 0.17 ± 0.01 a |
| Phenolic Alcohols | ||||
| Tyrosol | 17.97 ± 0.018 a | 14.67 ± 0.59 b | 1.57 ± 0.05 c | 1.07 ± 0.13 c |
| Hydroxytyrosol | 3.57 ± 0.02 b | 4.13 ± 0.11 a | 1.38 ± 0.20 c | 1.17 ± 0.12 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive Leaf Addition Increases Olive Oil Nutraceutical Properties. Molecules 2019, 24, 545. https://doi.org/10.3390/molecules24030545
Tarchoune I, Sgherri C, Eddouzi J, Zinnai A, Quartacci MF, Zarrouk M. Olive Leaf Addition Increases Olive Oil Nutraceutical Properties. Molecules. 2019; 24(3):545. https://doi.org/10.3390/molecules24030545
Chicago/Turabian StyleTarchoune, Imen, Cristina Sgherri, Jamel Eddouzi, Angela Zinnai, Mike Frank Quartacci, and Mokhtar Zarrouk. 2019. "Olive Leaf Addition Increases Olive Oil Nutraceutical Properties" Molecules 24, no. 3: 545. https://doi.org/10.3390/molecules24030545