Phosphonium-based Ionic Liquid Modified Activated Carbon from Mixed Recyclable Waste for Mercury(II) Uptake
Abstract
:1. Introduction
2. Results and Discussion
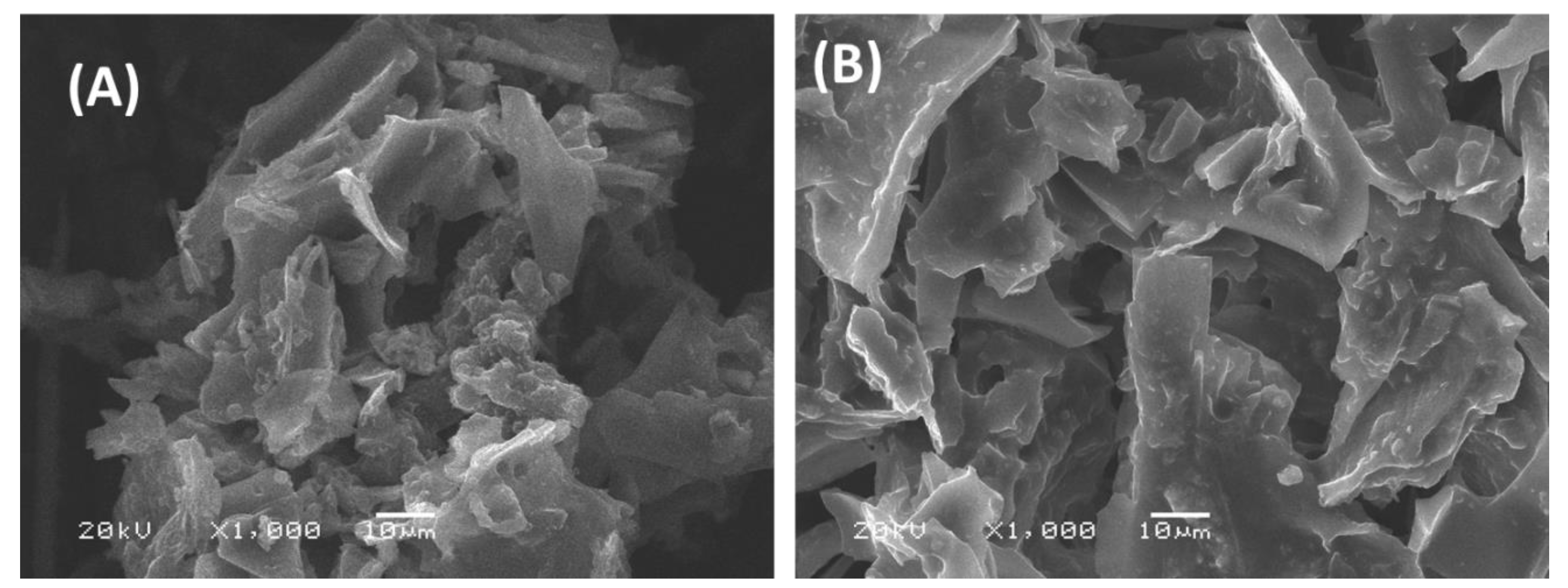
2.1. Characterization of the Modified Activated Carbon
2.2. Adsorption Studies
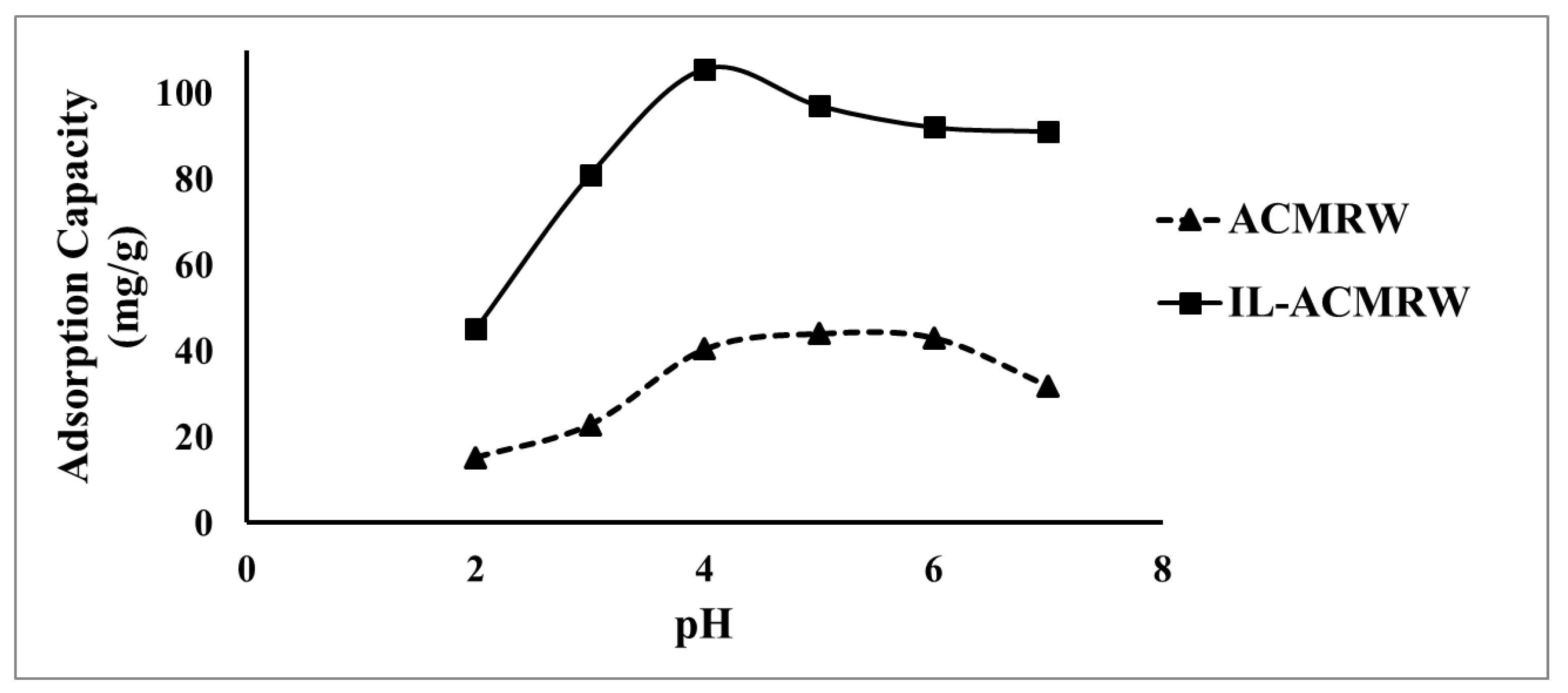
2.2.1. Effect of pH on the Hg(II) Removal
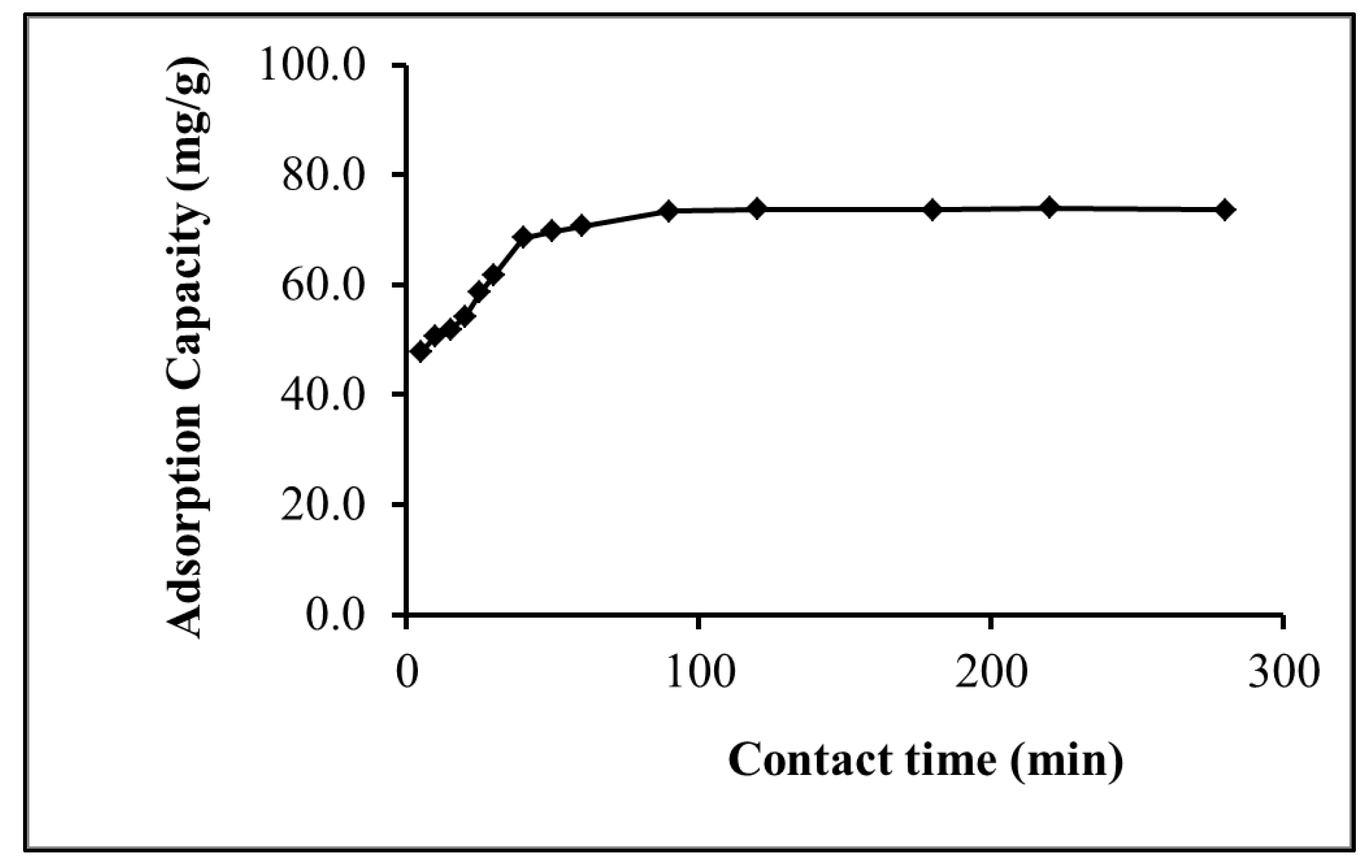
2.2.2. Effect of Contact Time
2.2.3. Kinetic Studies
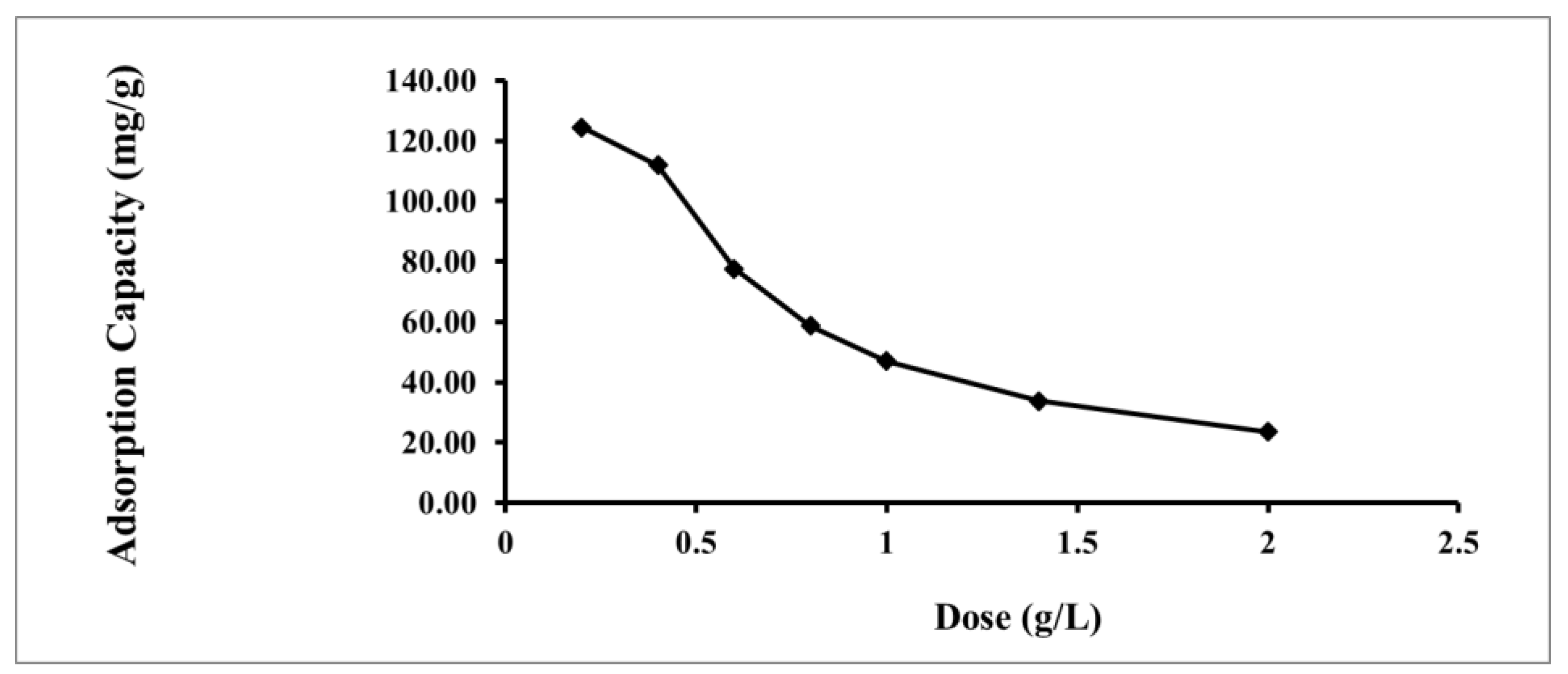
2.2.4. Effect of Adsorbent Dosage
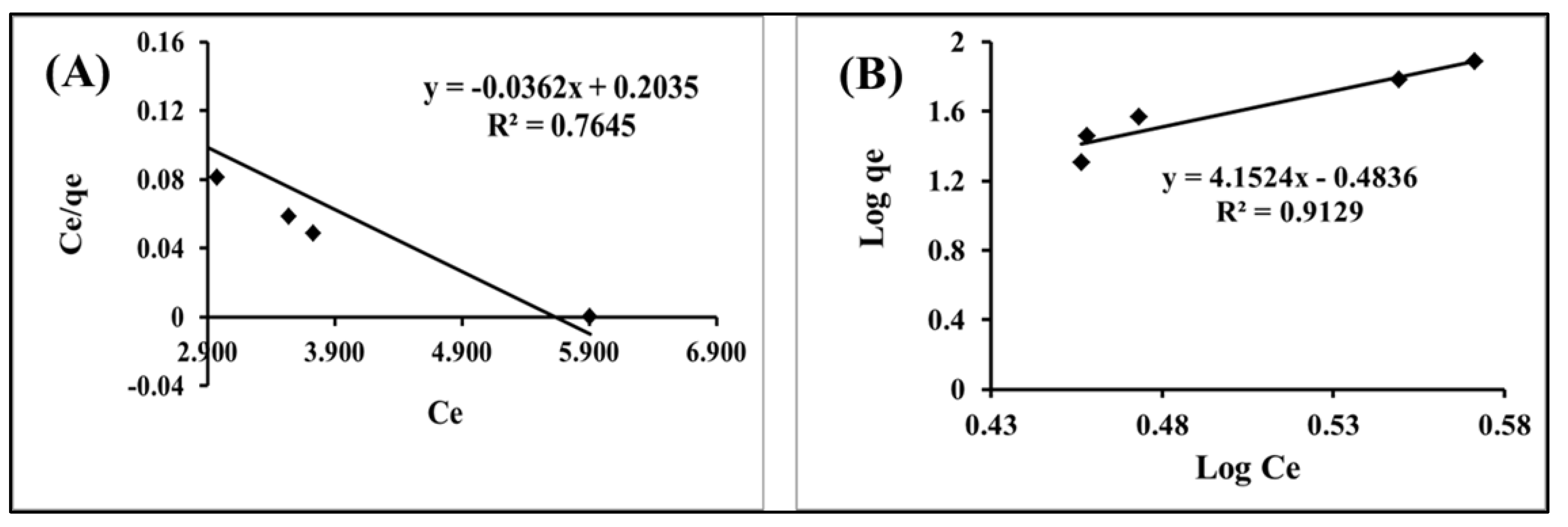
2.2.5. Adsorption Isotherms
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Trihexyl(tetradecyl)phosphonium Bis2,4,4-(trimethylpentyl) Phosphinate (Cyphos® IL 104) Modified Activated Carbon (IL-ACMRW)
3.3. Adsorption Studies of Hg(II) Onto IL-ACMRW
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byun, Y.; Koh, D.J.; Shin, D.N. Removal mechanism of elemental mercury by using non-thermal plasma. Chemosphere 2011, 83, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, J. Analytical methods for mercury in national pollutant discharge elimination systems (npdes) permits. O.o.W. Management. US EPA 2007, 1–4. [Google Scholar]
- Yang, S.; Yan, N.; Guo, Y.; Wu, D.; He, H.; Qu, Z.; Li, J.; Zhou, Q.; Jia, J. Gaseous Elemental Mercury Capture from Flue Gas Using Magnetic Nanosized (Fe3−xMnx)1−δO4. Environ. Sci. Technol. 2011, 45, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Gomez-Salazar, S.; Tavlarides, L.L. Mercury(II) Adsorption from Wastewaters Using a Thiol Functional Adsorbent. Ind. Eng. Chem. Res. 2003, 42, 1955–1964. [Google Scholar] [CrossRef]
- National primary drinking water standards. Report EPA 816-F-01-007, Washington DC. USEPA 2001.
- Luo, C.-S.; Huang, S.-D. Removal of Copper from Aqueous Amminecopper(II) Solution by Foam Flotation. Sep. Sci. Technol. 1993, 28, 1395–1408. [Google Scholar] [CrossRef]
- Henneberry, Y.K.; Kraus, T.E.C.; Fleck, J.A.; Krabbenhoft, D.P.; Bachand, P.M.; Horwath, W.R. Removal of inorganic mercury and methylmercury from surface waters following coagulation of dissolved organic matter with metal-based salts. Sci. Total Environ. 2011, 409, 631–637. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Yin, Y. Removal of Hg0 from flue gas using two homogeneous photo-fenton-like reactions. AIChE J. 2015, 61, 1322–1333. [Google Scholar] [CrossRef]
- Chiarle, S.; Ratto, M.; Rovatti, M. Mercury removal from water by ion exchange resins adsorption. Water Res. 2000, 34, 2971–2978. [Google Scholar] [CrossRef]
- Al Othman, Z.A.; Habila, M.A.; Hashem, A. Removal of zinc(II) from aqueous solutions using modified agricultural wastes: kinetics and equilibrium studies. Arabian J. Geosci. 2013, 6, 4245–4255. [Google Scholar] [CrossRef]
- AlOthman, Z.A.; Habila, M.A.; Ali, R.; Abdel Ghafar, A.; El-din Hassouna, M.S. Valorization of two waste streams into activated carbon and studying its adsorption kinetics, equilibrium isotherms and thermodynamics for methylene blue removal. Arabian J. Chem. 2014, 7, 1148–1158. [Google Scholar] [CrossRef] [Green Version]
- Johari, K.; Saman, N.; Mat, H. A comparative evaluation of mercury(II) adsorption equilibrium and kinetics onto silica gel and sulfur-functionalised silica gels adsorbents. The Canadian J. Chem. Eng. 2013, 92, 1048–1058. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Habila, M.A.; Alothman, Z.A.; Ali, R.; Ghafar, A.A.; Hassouna, M.S.E.-D. Removal of Tartrazine Dye onto Mixed-Waste Activated Carbon: Kinetic and Thermodynamic Studies. CLEAN—Soil Air Water 2013, 42, 1824–1831. [Google Scholar] [CrossRef]
- Aworn, A.; Thiravetyan, P.; Nakbanpote, W. Preparation and characteristics of agricultural Recyclable Waste Activated Carbon by physical activation having micro- and mesopores. J. Anal. Appl. Pyrol. 2008, 82, 279–285. [Google Scholar] [CrossRef]
- Zhang, F.-S.; Nriagu, J.O.; Itoh, H. Mercury removal from water using activated carbons derived from organic sewage sludge. Water Res. 2005, 39, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Vernon, J.D.; Bonzongo, J.-C.J. Volatilization and sorption of dissolved mercury by metallic iron of different particle sizes: Implications for treatment of mercury contaminated water effluents. J. Hazard. Mater. 2014, 276, 408–414. [Google Scholar] [CrossRef]
- Anoop Krishnan, K.; Anirudhan, T.S. Removal of mercury(II) from aqueous solutions and chlor-alkali industry effluent by steam activated and sulphurised activated carbons prepared from bagasse pith: kinetics and equilibrium studies. J. Hazard. Mater. 2002, 92, 161–183. [Google Scholar] [CrossRef]
- Yardim, M.F.; Budinova, T.; Ekinci, E.; Petrov, N.; Razvigorova, M.; Minkova, V. Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural. Chemosphere 2003, 52, 835–841. [Google Scholar] [CrossRef]
- Gupta, A.; Vidyarthi, S.R.; Sankararamakrishnan, N. Enhanced sorption of mercury from compact fluorescent bulbs and contaminated water streams using functionalized multiwalled carbon nanotubes. J. Hazard. Mater. 2014, 274, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Sui, J.; Li, J.; Tang, Y.; Cai, W. Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes. Chem. Eng. J. 2012, 210, 45–52. [Google Scholar] [CrossRef]
- Chen, P.H.; Hsu, C.-F.; Tsai, D.D.-W.; Lu, Y.-M.; Huang, W.-J. Adsorption of mercury from water by modified multi-walled carbon nanotubes: adsorption behaviour and interference resistance by coexisting anions. Environ. Technol. 2014, 35, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Ganzagh, M.A.A.; Yousefpour, M.; Taherian, Z. The removal of mercury(II) from water by Ag supported on nanomesoporous silica. J. Chem. Biol. 2016, 9, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, U.A.; Solangi, A.R.; Memon, S.Q.; Taqvi, S.I.H.; Memon, N. Ionic Liquid Modified Resin for the Adsorptive Removal of Dibutyl Phthalate: Equilibrium, Kinetic, and Thermodynamic Studies. CLEAN—Soil Air Water 2012, 40, 630–639. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Chemistry. Ionic liquids—Solvents of the uture? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Bosmann, A.; Datsevich, L.; Jess, A.; Lauter, A.; Schmitz, C.; Wasserscheid, P. Deep desulfurization of diesel fuel by extraction with ionic liquids. Chem. Commun. 2001, 23, 2494–2495. [Google Scholar] [CrossRef]
- Al Othman, Z.A.; Hashem, A.; Habila, M.A. Kinetic, Equilibrium and Thermodynamic Studies of Cadmium(II) Adsorption by Modified Agricultural Wastes. Molecules 2011, 16, 10443–10456. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. The Canadian J. Chem. Eng. 2009, 76, 822–827. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Ibrahim, M.A.; Labis, J.P.; AlOthman, Z.A. Simple and facile synthesis of amino functionalized hollow core-mesoporous shell silica spheres using anionic surfactant for Pb(II), Cd(II), and Zn(II) adsorption and recovery. Chem. Eng. J. 2014, 251, 441–451. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Hutson, N.D.; Yang, R.T. Theoretical basis for the Dubinin-Radushkevitch (D-R) adsorption isotherm equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Freundlich, H. Uber Die Adsorption in Lösungen. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Jassam, T.M.; Akib, S.; Ali Hashim, M. Novel deep eutectic solvent-functionalized carbon nanotubes adsorbent for mercury removal from water. J. Colloid Interf. Sci. 2017, 497, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.; Yilmaz, E.; Alothman, Z.A.; Soylak, M. Flame atomic absorption spectrometric determination of Cd, Pb, and Cu in food samples after pre-concentration using 4-(2-thiazolylazo) resorcinol-modified activated carbon. J. Ind. Eng. Chem. 2014, 20, 3989–3993. [Google Scholar] [CrossRef]
- Yusuf, N.Y.; Masdar, M.S.; Isahak, W.N.R.W.; Nordin, D.; Husaini, T.; Majlan, E.H.; Rejab, S.A.M.; Chew, C.L. Ionic liquid-impregnated activated carbon for biohydrogen purification in an adsorption unit. Presented at the 29th Symposium of Malaysian Chemical Engineers (SOMChE), Miri Sarwak, Malaysia, 1–3 December 2016. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds including; activated carbon from mixed recyclable waste (ACMRW) and ionic liquid modified activated carbon from mixed recyclable waste (IL-ACMRW) are available from the authors. |


| The Pseudo-First-Order | The Pseudo-Second-Order | ||||
|---|---|---|---|---|---|
| Rate | qe (mg/g) | Correlation | Rate | qe (mg/g) | Correlation |
| constant | coefficient | constant | coefficient | ||
| (K1) | (R21) | (K2) | (R22) | ||
| 0.046 | 39.3 | 0.96 | - | 73.5 | 0.99 |
| Langmuir Constants | Constant | In the case of Hg(II) adsorption onto IL-ACMRW |
| KL | 4.885 | |
| b | 0.177 | |
| Qmax | 27.6 | |
| R2 | 0.764 | |
| Freundlich Constants | KF | 0.32 |
| n | 0.24 | |
| R2 | 0.912 |
| Adsorbent | qmax (mg/g) | Reference |
|---|---|---|
| Trihexyl(tetradecyl)phosphonium Bis2,4,4-(trimethylpentyl)phosphinate modified activated carbon from mixed recyclable waste (IL-ACMRW) | 124 | Current work |
| Sulfur incorporated multiwall carbon nanotube | 151.5 | [21] |
| Thiolated multiwall carbon nanotube | 84.66 | [22] |
| Thiol-functionalized superparamagnetic carbon nanotubes | 65.52 | [23] |
| Carboxylic modified multiwall carbon nanotube | 127.6 | [24] |
| Ag supported on nanomesoporous silica | 42.26 | [25] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habila, M.A.; AlOthman, Z.A.; Ghfar, A.A.; Al-Zaben, M.I.M.; Alothman, A.A.S.; Abdeltawab, A.A.; El-Marghany, A.; Sheikh, M. Phosphonium-based Ionic Liquid Modified Activated Carbon from Mixed Recyclable Waste for Mercury(II) Uptake. Molecules 2019, 24, 570. https://doi.org/10.3390/molecules24030570
Habila MA, AlOthman ZA, Ghfar AA, Al-Zaben MIM, Alothman AAS, Abdeltawab AA, El-Marghany A, Sheikh M. Phosphonium-based Ionic Liquid Modified Activated Carbon from Mixed Recyclable Waste for Mercury(II) Uptake. Molecules. 2019; 24(3):570. https://doi.org/10.3390/molecules24030570
Chicago/Turabian StyleHabila, Mohamed A., Zeid A. AlOthman, Ayman A. Ghfar, Maha I.M. Al-Zaben, Ahmed A.S. Alothman, Ahmed A. Abdeltawab, Adel El-Marghany, and Mohamed Sheikh. 2019. "Phosphonium-based Ionic Liquid Modified Activated Carbon from Mixed Recyclable Waste for Mercury(II) Uptake" Molecules 24, no. 3: 570. https://doi.org/10.3390/molecules24030570







