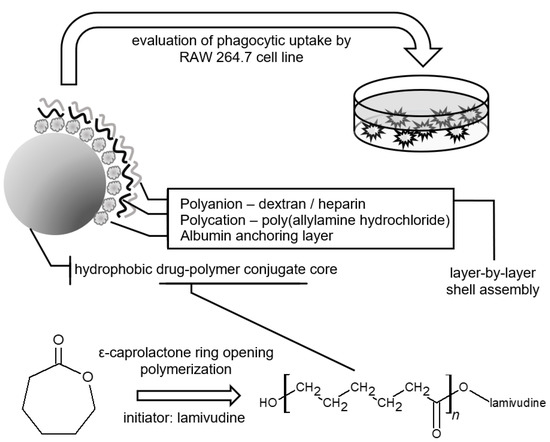
Microparticles of Lamivudine—Poly-ε-Caprolactone Conjugate for Drug Delivery via Internalization by Macrophages
Abstract
:1. Introduction
2. Results
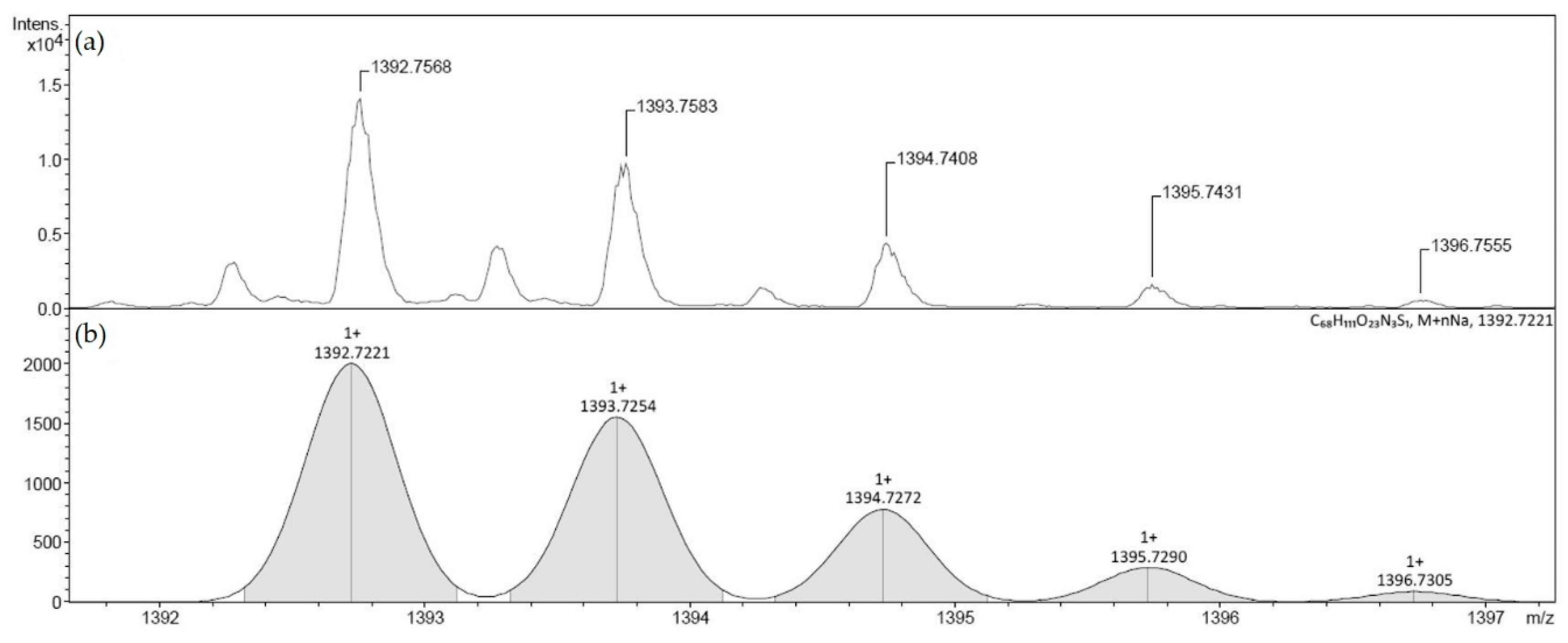
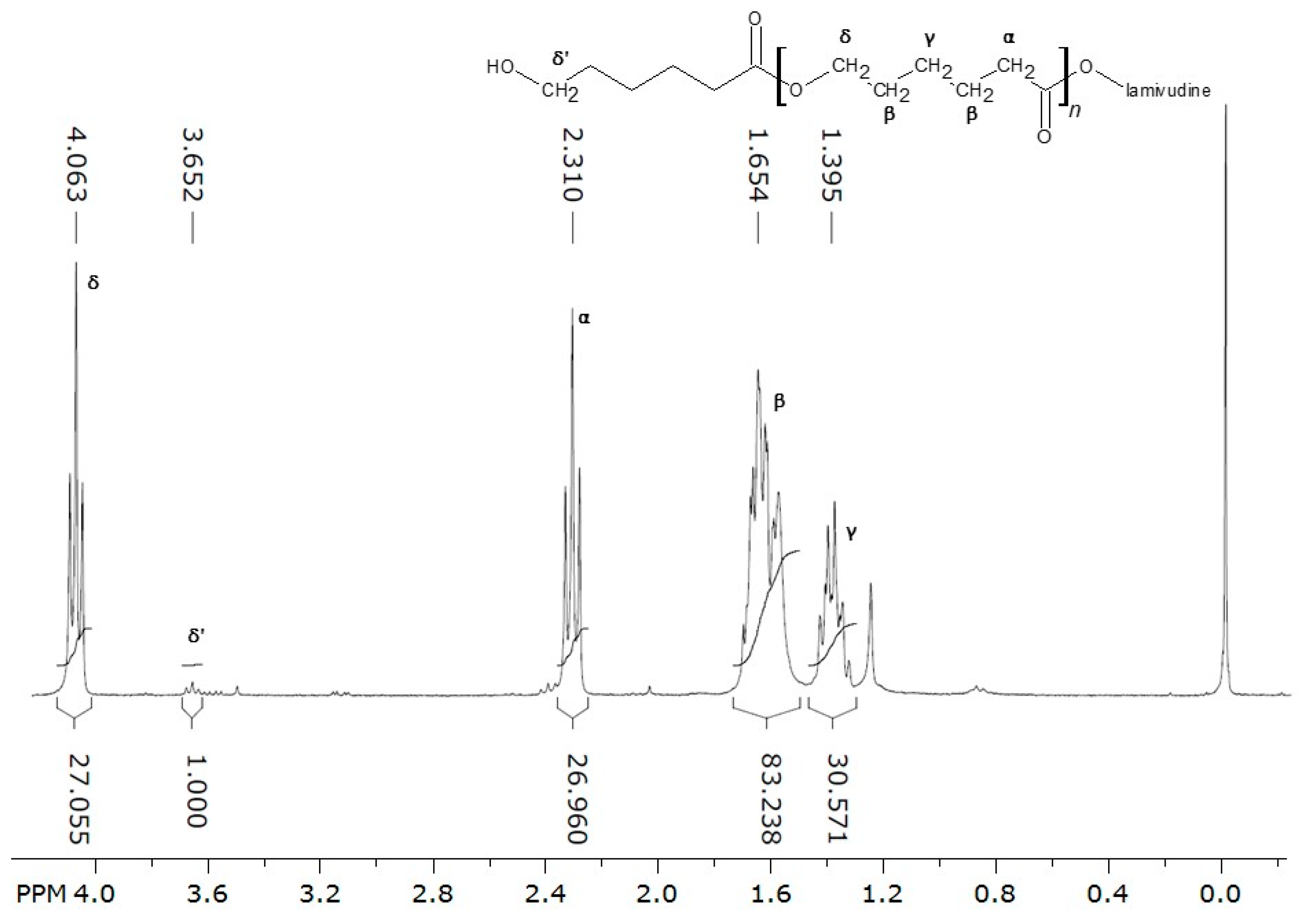
2.1. Conjugate Synthesis
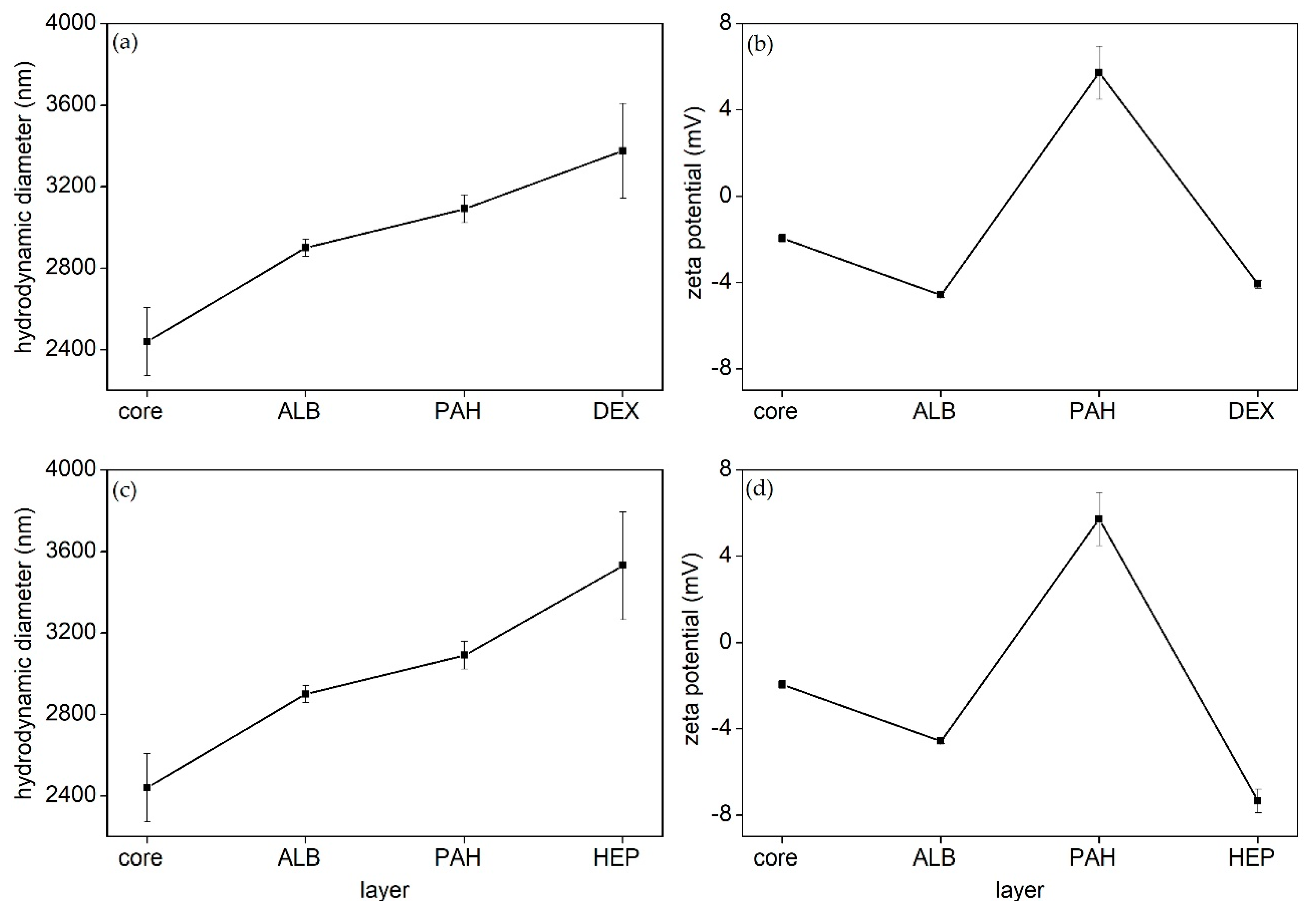
2.2. LbL Shell Assembly and Particle Characterization
2.3. Scanning Electron Microscopy Imaging
2.4. Phagocytosis In Vitro Assay
2.5. In Vitro Viability Assay
3. Discussion
3.1. Conjugate Synthesis
3.2. Microparticle Formation and LbL Shell Assembly
3.3. In Vitro Phagocytosis assay
4. Materials and Methods
4.1. Materials
4.2. Conjugate Synthesis
4.3. Microsphere Preparation
4.4. Layer-by-Layer Shell Assembly
4.5. Product Characterization
4.6. Cell Culture
4.7. Quantification of Intracellular Trafficking of Fluorescently Labeled Particles via Flow Cytometry
4.8. Cell Viability Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2017, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H.; Grainger, D. Nanoparticle Uptake: The Phagocyte Problem HHS Public Access. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Alexandru-flaviu, T.; Cornel, C. Macrophages Targeted Drug Delivery as a Key Therapy in Infectious Disease. Biotechnol. Mol. Biol. Nanomed. 2014, 2, 19–21. [Google Scholar]
- Leavy, O. Immunotherapy: Stopping Monocytes in Their Tracks. Nat. Rev. Immunol. 2011, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Nakagawa, K.; Sugihara, F.; Kuwahara, R.; Ashihara, M.; Yamane, F.; Minowa, Y.; Fukushima, K.; Ebina, I.; Yoshioka, Y.; et al. Identification of an Atypical Monocyte and Committed Progenitor Involved in Fibrosis. Nature 2016, 541, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Jimenez, S.A. The Significance of Macrophage Polarization Subtypes for Animal Models of Tissue Fibrosis and Human Fibrotic Diseases. Clin. Transl. Med. 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.R.; Cottam, M.A.; Kennedy, A.J.; Hasty, A.H. Macrophage-Targeted Therapeutics for Metabolic Disease. Trends Pharmacol. Sci. 2018, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Milan, D.J.; Ellinor, P.T.; Nahrendorf, M.; Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; et al. Macrophages Facilitate Electrical Conduction in the Heart Article Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Curley, P.; Liptrott, N.J.; Owen, A. Advances in Nanomedicine Drug Delivery Applications for HIV Therapy. Future Sci. OA. 2018, 4, FSO230. [Google Scholar] [CrossRef]
- Nowacek, A.S.; Miller, R.L.; McMillan, J.; Kanmogne, G.; Kanmogne, M.; Mosley, R.L.; Ma, Z.; Graham, S.; Chaubal, M.; Werling, J.; et al. NanoART Synthesis, Characterization, Uptake, Release and Toxicology for Human Monocyte-Macrophage Drug Delivery. Nanomedicine (Lond.) 2009, 4, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Agashe, H.B.; Garg, M.; Balasubramanium, P.; Kabra, M.; Jain, N.K. Poly (Propyleneimine) Dendrimer Based Nanocontainers for Targeting of Efavirenz to Human Monocytes/Macrophages in Vitro. J. Drug Target. 2007, 15, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, T.; Hirshkowitz, A. Optimal Structural Design of Mannosylated Nanocarriers for Macrophage Targeting Peiming. J. Control. Release 2015, 85, 1–27. [Google Scholar]
- Li, H.; Tatematsu, K.; Somiya, M.; Iijima, M.; Kuroda, S. Development of a Macrophage-Targeting and Phagocytosis-Inducing Bio-Nanocapsule-Based Nanocarrier for Drug Delivery. Acta Biomater. 2018, 73, 412–423. [Google Scholar] [CrossRef]
- Pacheco, P.; White, D.; Sulchek, T. Effects of Microparticle Size and Fc Density on Macrophage Phagocytosis. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Schöttler, S.; Klein, K.; Landfester, K.; Mailänder, V. Protein Source and Choice of Anticoagulant Decisively Affect Nanoparticle Protein Corona and Cellular Uptake. Nanoscale 2016, 8, 5526–5536. [Google Scholar] [CrossRef]
- Larson, N.; Ghandeharia, H. Polymeric Conjugates for Drug Delivery. Adv. Quantum Chem. 2012, 24, 840–853. [Google Scholar] [CrossRef]
- Pelegri-Oday, E.M.; Lin, E.W.; Maynard, H.D. Therapeutic Protein-Polymer Conjugates: Advancing beyond Pegylation. J. Am. Chem. Soc. 2014, 136, 14323–14332. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Woodward, S.C.; Brewer, P.S.; Moatamed, F.; Schindler, A.; Pitt, C.G. The Intracellular Degradation of Poly (Ε-caprolactone). J. Biomed. Mater. Res. 1985, 19, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, L. Ring-Opening Polymerization of E-Caprolactone Initiated by Natural Amino Acids. Macromolecules 2004, 37, 2674–2676. [Google Scholar] [CrossRef]
- Storey, R.F.; Sherman, J.W. Kinetics and Mechanism of the Stannous Octoate-Catalyzed Bulk Polymerization of E-Caprolactone. Macromolecules 2002, 35, 1504–1512. [Google Scholar] [CrossRef]
- He, Y.; Park, K. Effects of the Microparticle Shape on Cellular Uptake. Mol. Pharm. 2016, 13, 2164–2171. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Rathmann, S.; Schönberg, M.; Leßig, J.; Reibetanz, U. Interaction, Uptake, and Processing of LbL-Coated Microcarriers by PMNs. Cytom. Part A 2011, 79, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Polomska, A.; Leroux, J.C.; Brambilla, D. Layer-by-Layer Coating of Solid Drug Cores: A Versatile Method to Improve Stability, Control Release and Tune Surface Properties. Macromol. Biosci. 2017, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Rühl, D.; Lück, M.; Paulke, B.R. Influence of Fluorescent Labelling of Polystyrene Particles on Phagocytic Uptake, Surface Hydrophobicity, and Plasma Protein Adsorption. Pharm. Res. 1997, 14, 18–24. [Google Scholar]
- Tabata, Y.; Ikada, Y. Effect of the Size and Surface Charge of Polymer Microspheres on Their Phagocytosis by Macrophage. Biomaterials 1988, 9, 356–362. [Google Scholar] [CrossRef]
- Gao, H.; Shi, W.; Freund, L.B. Mechanics of Receptor-Mediated Endocytosis. Proc. Natl. Acad. Sci. USA. 2005, 102, 9469–9474. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR Study of Polycaprolactone Chain Organization at Interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of Scanning Electron Microscopy, Dynamic Light Scattering and Analytical Ultracentrifugation for the Sizing of Poly(Butyl Cyanoacrylate) Nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Yoshioka, R.; Nakashima, Y.; Fujiwara, Y.; Komohara, Y.; Takeya, M.; Nakanishi, Y. The Biological Response of Macrophages to PMMA Particles with Different Morphology and Size. Biosurf. Biotribol. 2016, 2, 114–120. [Google Scholar] [CrossRef]
- Thiele, L.; Merkle, H.P.; Walter, E. Phagocytosis and Phagosomal Fate of Surface-Modified Microparticles in Dendritic Cells and Macrophages. Pharm. Res. 2003, 20, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.; Walker, A.; Mitragotri, S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Đapić, I.; Kovačević, D. Electrokinetic Study of Bovine Serum Albumin Adsorption on Previously Formed PAH/PSS Multilayer. Croat. Chem. Acta 2011, 84, 185–191. [Google Scholar] [CrossRef]
- Unterweger, H.; Janko, C.; Schwarz, M.; Dézsi, L.; Urbanics, R.; Matuszak, J.; Őrfi, E.; Fülöp, T.; Bäuerle, T.; Szebeni, J.; et al. Non-Immunogenic Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles: A Biocompatible, Size-Tunable Contrast Agent for Magnetic Resonance Imaging. Int. J. Nanomed. 2017, 12, 5223–5238. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Ji, J.; Yuan, W.; Shen, J. Construction of Anti-Adhesive and Antibacterial Multilayer Films via Layer-by-Layer Assembly of Heparin and Chitosan. Biomaterials 2005, 26, 6684–6692. [Google Scholar] [CrossRef]
- Falcone, D.J. Heparin Stimulation of Plasminogen Activator Secretion by Macrophage-like Cell Line Raw264.7: Role of the Scavenger Receptor. J. Cell. Physiol. 1989, 140, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bleiberg, I.; MacGregor, I.; Aronsona, M. Heparin Receptors on Mouse Macrophages. Thromb. Res. 1983, 29, 53–61. [Google Scholar] [CrossRef]
- Haddad, M.; Laghzaoui, M.; Welter, R.; Dagorne, S. Synthesis and Structure of Neutral and Cationic Aluminum Complexes Supported by Bidentate O,P-Phosphinophenolate Ligands and Their Reactivity with Propylene Oxide and ε-Caprolactone. Organometallics 2009, 28, 4584–4592. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbaniak, T.; Machová, D.; Janoušková, O.; Musiał, W. Microparticles of Lamivudine—Poly-ε-Caprolactone Conjugate for Drug Delivery via Internalization by Macrophages. Molecules 2019, 24, 723. https://doi.org/10.3390/molecules24040723
Urbaniak T, Machová D, Janoušková O, Musiał W. Microparticles of Lamivudine—Poly-ε-Caprolactone Conjugate for Drug Delivery via Internalization by Macrophages. Molecules. 2019; 24(4):723. https://doi.org/10.3390/molecules24040723
Chicago/Turabian StyleUrbaniak, Tomasz, Daniela Machová, Olga Janoušková, and Witold Musiał. 2019. "Microparticles of Lamivudine—Poly-ε-Caprolactone Conjugate for Drug Delivery via Internalization by Macrophages" Molecules 24, no. 4: 723. https://doi.org/10.3390/molecules24040723