Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies
Abstract
:1. Introduction
2. Fabrication of Electrospun Polymer Nanofibers
2.1. The Basic Setup and Composition for Electrospinning
2.2. Mechanism of Electrospinning
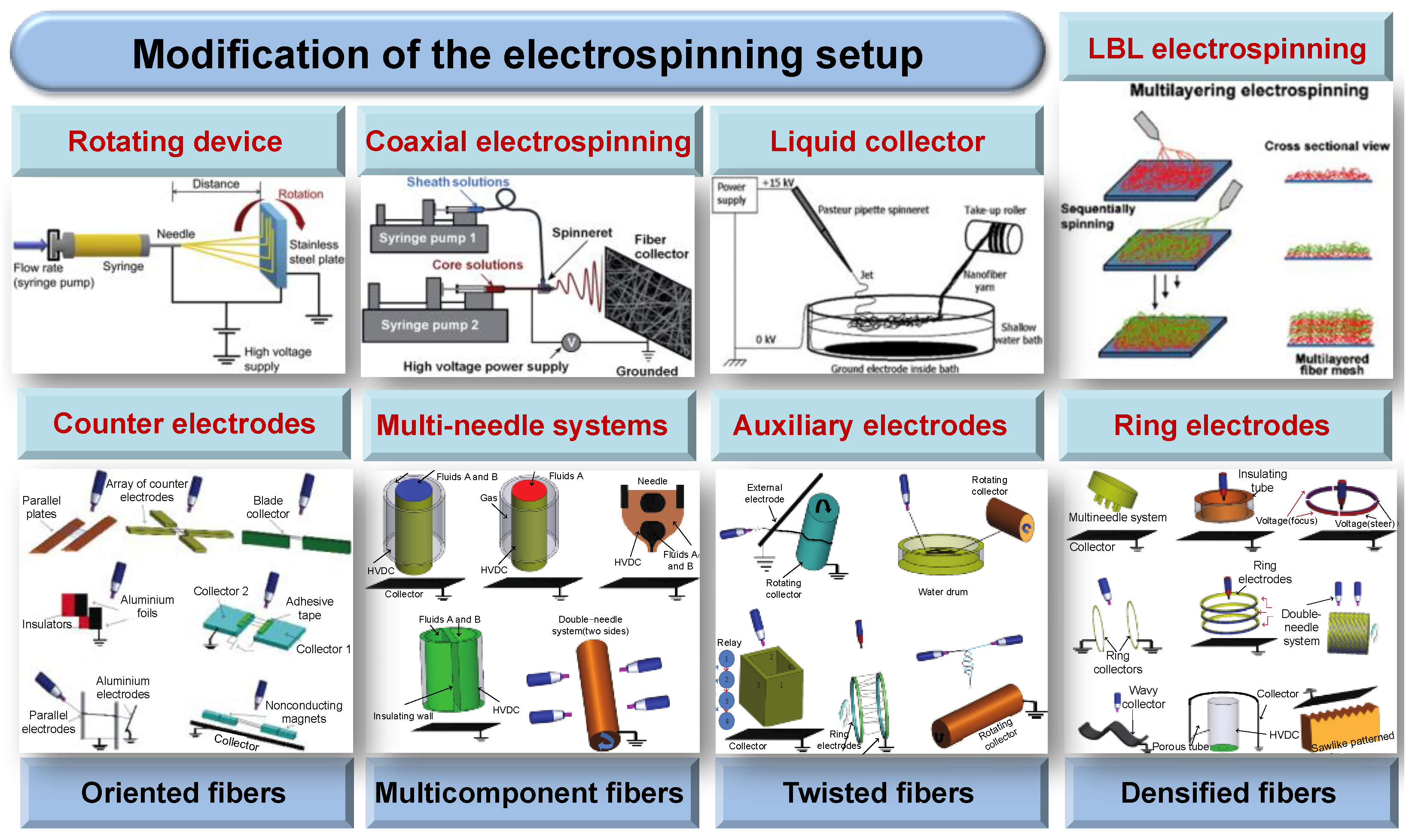
2.3. Modification of the Electrospinning Setup
2.4. Electrospinning Methods
3. Diverse Morphologies of Electrospun Polymer Nanofibers
3.1. Core/Shell Structures
3.2. Hollow Interiors
3.3. Porous Structures
3.4. Multilayer Structures
3.5. Side-by-Side Structures
4. Challenges and Future Perspectives of Electrospun Polymer Nanofibers
Author Contributions
Funding
Conflicts of Interest
References
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Saeed, K.; Haider, S.; Oh, T.J.; Park, S.Y. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption. J. Membr. Sci. 2008, 322, 400–405. [Google Scholar] [CrossRef]
- Formo, E.; Lee, E.; Campbell, D.; Xia, Y. Functionalization of electrospun TiO2 nanofibers with Pt nanoparticles and nanowires for catalytic applications. Nano Lett. 2008, 8, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous materials and their applications. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S. Applications of polymer nanofibers in biomedicine and biotechnology. Appl. Biochem. Biotechnol. 2005, 125, 147–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tang, Y.; Vlahovic, B.; Yan, F. Electrospun polymer nanofibers decorated with noble metal nanoparticles for chemical sensing. Nanoscale Res. Lett. 2017, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.J.; Phatak, A.; Giles, D.W.; Macosko, C.W.; Bates, F.S. Melt blown nanofibers: Fiber diameter distributions and onset of fiber breakup. Polymer 2007, 48, 3306–3316. [Google Scholar] [CrossRef]
- Barhate, R.S.; Ramakrishna, S. Nanofibrous filtering media: Filtration problems and solutions from tiny materials. J. Membr. Sci. 2007, 296, 1–8. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, B.S.; Yoo, Y.C.; Khil, M.S.; Kim, H.Y. Enhanced mechanical properties of multilayer nano-coated electrospun nylon 6 fibers via a layer-by-layer self-assembly. J. Appl. Polym. Sci. 2008, 107, 2211–2216. [Google Scholar] [CrossRef]
- Tiwari, A.; Terada, D.; Yoshikawa, C.; Kobayashi, H. An enzyme-free highly glucose-specific assay using self-assembled aminobenzene boronic acid upon polyelectrolytes electrospun nanofibers-mat. Talanta 2010, 82, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, Y.T.; Cho, S.; Song, W.J.; Moon, S.; Park, C.G.; Park, S.; Myoung, J.M.; Jeong, U. Surface-embedded stretchable electrodes by direct printing and their uses to fabricate ultrathin vibration sensors and circuits for 3D structures. Adv. Mater. 2017, 29, 1702625. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L. Ultrastretchable and self-healing double-network hydrogel for 3D printing and strain sensor. ACS Appl. Mater. Interfaces 2017, 9, 26429–26437. [Google Scholar] [CrossRef] [PubMed]
- McCullen, S.D.; Stevens, D.R.; Roberts, W.A.; Ojha, S.S.; Clarke, L.I.; Gorga, R.E. Morphological, electrical, and mechanical characterization of electrospun nanofiber mats containing multiwalled carbon nanotubes. Macromolecules 2007, 40, 997–1003. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Wang, Z.; Crandall, C.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Microfiltration performance of electrospun nanofiber membranes with varied fiber diameters and different membrane porosities and thicknesses. Polymer 2017, 114, 64–72. [Google Scholar] [CrossRef]
- Formhals, A. Process and Apparatus for Preparing Artificial Threads. U.S. Patent No. 1975504, 1934. Available online: https://patents.glgoo.top/patent/US1975504A/en (accessed on 2 October 2018).
- Doshi, J.; Reneker, D.H. Electrospinning Process and Applications of Electrospun Fibers, Industry Applications Society Annualmeeting, 1993, Conference Record of the 1993 IEEE, 1993. IEEE: 1698–1703. Available online: https://ieeexplore.ieee.org/document/299067/metrics#metrics (accessed on 2 October 2018).
- Tan, S.H.; Inai, R.; Kotaki, M.; Ramakrishna, S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 2005, 46, 6128–6134. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.H.; Liu, P.; He, J.H. Tunable surface morphology of electrospun PMMA fiber using binary solvent. Appl. Surf. Sci. 2016, 364, 516–521. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, D.W.; Nam, K.B.; Gim, Y.H.; Ko, H.S.; Seo, D.K.; Lee, G.H.; Kim, Y.H.; Kim, S.W.; Oh, T.S.; et al. Continuous bundles of aligned electrospun PAN nano-fiber using electrostatic spiral collector and converging coil. Polymer 2016, 84, 52–58. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Joshi, M.K.; Kim, J.I.; Unnithan, A.R.; Lee, J.; Park, C.H.; Kim, C.S. Bimodal fibrous structures for tissue engineering: Fabrication, characterization and in vitro biocompatibility. J. Colloid Interface Sci. 2016, 476, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Okoli, J.U.; Briggs, T.A.; Major, I.E. Application of nanotechnology in the manufacturing sector: A review. Niger. J. Technol. 2013, 32, 379–385. [Google Scholar]
- Park, S.H.; Kim, T.G.; Kim, H.C.; Yang, D.Y.; Park, T.G. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomater. 2008, 4, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Arinzeh, T.L.; Weber, N.; Jaffe, M. Electrospun Electroactive Polymers for Regenerative Medicine Applications. U.S. Patent No. 10052412, 2017. Available online: https://patents.justia.com/patent/10052412 (accessed on 21 August 2018).
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q.; Li, Z. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215. [Google Scholar] [PubMed] [Green Version]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Ji, Y.; Li, B.; Ge, S.; Sokolov, J.C.; Rafailovich, M.H. Structure and nanomechanical characterization of electrospun PS/Clay nanocomposite fibers. Langmuir 2006, 22, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bellan, L.M.; Craighead, H.G.; Frey, M.W. Formation and properties of nylon-6 and nylon-6/montmorillonite composite nanofibers. Polymer 2006, 47, 6208–6217. [Google Scholar] [CrossRef]
- Ridolfi, D.M.; Lemes, A.P.; de Oliveira, S.; Justo, G.Z.; Palladino, M.V.; Durán, N. Electrospun poly(ethylene oxide)/chitosan nanofibers with cellulose nanocrystals as support for cell culture of 3T3 fibroblasts. Cellulose 2017, 24, 3353–3365. [Google Scholar] [CrossRef]
- Chew, S.Y.; Wen, J.; Yim, E.K.F.; Leong, K.W. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules 2005, 6, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Wannatong, L.; Sirivat, A.; Supaphol, P. Effects of solvents on electrospun polymeric fibers: Preliminary study on polystyrene. Polym. Int. 2004, 53, 1851–1859. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Pham, Q.P.; Sharma, U.; Mikos, D.A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.L.; Yu, S.H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Kong, L.; Li, L.; Li, N.; Yan, P. Antitumor activity of doxorubicin-loaded carbon nanotubes incorporated poly(lactic-co-glycolic acid) electrospun composite nanofibers. Nanoscale Res. Lett. 2015, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Martins, P.; Moya, X.; Ghidini, M.; Sencadas, V.; Botelho, G.; Mathur, N.D.; Lanceros-Mendez, S. Magnetoelectric CoFe2O4/polyvinylidene fluoride electrospun nanofibres. Nanoscale 2015, 7, 8058–8061. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Yamamoto, E.; Mannen, T.; Nagamune, T.; Nagamune, T. Protein refolding using chemical refolding additives. Biotechnol. J. 2013, 8, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; He, M.; Liu, H.; Niu, Y.; Crawford, A.; Coates, P.D.; Chen, D.; Shi, R.; Zhang, L. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014, 35, 9395–9405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.Y.; Chang, C.C.; Cheng, L.P. Preparation of hydrophobic nanofibers by electrospinning of PMMA dissolved in 2-propanol and water. MATEC Web Conf. 2019, 264, 03004. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Gonzales, R.R.; Abdel Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Fornaguera, C.; Dols Perez, A.; Calderó, G.; García Celma, M.J.; Camarasa, J.; Solans, C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood–brain barrier. J. Controlled Release 2015, 211, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Suwantong, O. Biomedical applications of electrospun polycaprolactone fiber mats. Polym. Adv. Technol. 2016, 27, 1264–1273. [Google Scholar] [CrossRef]
- Potrč, T.; Baumgartner, S.; Roškar, R.; Planinšek, O.; Lavrič, Z.; Kristl, J.; Kocbek, P. Electrospun polycaprolactone nanofibers as a potential oromucosal delivery system for poorly water-soluble drugs. Eur. J. Pharm. Sci. 2015, 75, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.A.M.; Silva, D.M.; Gomes, P.S.; Fernandes, M.H.; Santos, J.D.; Sencadas, V. Effect of sterilization methods on electrospun poly(lactic acid) (PLA) Fiber alignment for biomedical applications. ACS Appl. Mater. Interfaces 2016, 8, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Adhikary, P.; Jana, S.; Biswas, A.; Sencadas, V.; Gupta, S.D.; Tudu, B.; Mandal, D. Electrospun gelatin nanofiber based self-powered bio-e-skin for health care monitoring. Nano Energy 2017, 36, 166–175. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Al Masry, W.; Al Zeghayer, Y.; Al Hoshan, M.; Ali, F. Fabrication of chitosan nanofibers membrane via electrospinning. TechConnect Briefs 2011, 1, 810–812. [Google Scholar]
- Wang, W.; Jin, X.; Zhu, Y.; Zhu, C.; Yang, J.; Wang, H.; Lin, T. Effect of vapor-phase glutaraldehyde crosslinking on electrospun starch fibers. Carbohydr. Polym. 2016, 140, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.P.; Shanmugasundaram, S.; Masih, P.; Pandya, D.; Amara, S.; Collins, G.; Arinzeh, T.L. An investigation of common crosslinking agents on the stability of electrospun collagen scaffolds. J. Biomed. Mater. Res. Part A 2015, 103, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Sahoo, S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. Today 2017, 22, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, Z.; Bayrami, S.; Dorkoosh, F.A.; Akbari Javar, H.; Seyedjafari, E.; Zargarian, S.S.; Haddadi Asl, V. Development and characterization of electrosprayed nanoparticles for encapsulation of Curcumin. J. Biomed. Mater. Res. Part A 2018, 106, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand Ghayeni, M.; Naderi Meshkin, H.; Mahdizadeh, A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater. Sci. Eng. C 2016, 66, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Fukunishi, T.; Best, C.A.; Sugiura, T.; Shoji, T.; Yi, T.; Udelsman, B.; Ohst, D.; Ong, C.S.; Zhang, H.; Shinoka, T.; et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/Chitosan scaffolds in a sheep model. PLoS ONE 2016, 11, e0158555. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Rafii, A.; Biazar, E.; Heidari Keshel, S. Cellular response of limbal stem cells on PHBV/Gelatin nanofibrous scaffold for ocular epithelial regeneration. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 879–887. [Google Scholar] [CrossRef]
- Choi, M.O.; Kim, Y.J. Effect of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin ratios on the characteristics of biomimetic composite nanofibrous scaffolds. Colloid Polym. Sci. 2018, 296, 917–926. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 58, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Sessini, V.; Navarro-Baena, I.; Arrieta, M.P.; Dominici, F.; López, D.; Torre, L.; Kenny, J.M.; Dubois, P.; Raquez, J.M.; Peponi, L. Effect of the addition of polyester-grafted-cellulose nanocrystals on the shape memory properties of biodegradable PLA/PCL nanocomposites. Polym. Degrad. Stab. 2018, 152, 126–138. [Google Scholar] [CrossRef]
- Saberi, A.; Rafienia, M.; Poorazizi, E. A novel fabrication of PVA/Alginate-Bioglass electrospun for biomedical engineering application. Nanomed. J. 2017, 4, 152–163. [Google Scholar]
- İspirli Doğaç, Y.; Deveci, İ.; Mercimek, B.; Teke, M. A comparative study for lipase immobilization onto alginate based composite electrospun nanofibers with effective and enhanced stability. Int. J. Biol. Macromol. 2017, 96, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Dhand, C.; Barathi, V.A.; Ong, S.T.; Venkatesh, M.; Harini, S.; Dwivedi, N.; Goh, E.T.L.; Nandhakumar, M.; Venugopal, J.R.; Diaz, S.M.; et al. Latent oxidative polymerization of catecholamines as potential cross-linkers for biocompatible and multifunctional biopolymer scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 32266–32281. [Google Scholar] [CrossRef] [PubMed]
- PranavKumar Shadamarshan, R.; Balaji, H.; Rao, H.S.; Balagangadharan, K.; Viji Chandran, S.; Selvamurugan, N. Fabrication of PCL/PVP electrospun fibers loaded with trans-anethole for bone regeneration in vitro. Colloids Surf. B 2018, 171, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Sasikala, A.R.K.; Murugesan, P.; Gurusamy, M.; Wu, D.; Park, C.H.; Kim, C.S. Electrospun polyurethane-dextran nanofiber mats loaded with Estradiol for post-menopausal wound dressing. Int. J. Biol. Macromol. 2015, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, B.; Mondal, B.; Ray, S.K.; Sarkar, S.C. A novel biocompatible conducting polyvinyl alcohol (PVA)-polyvinylpyrrolidone (PVP)-hydroxyapatite (HAP) composite scaffolds for probable biological application. Colloids Surf. B 2016, 143, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Simon, P.; Kim, J.S. Electrospun PVA/HAp nanocomposite nanofibers: Biomimetics of mineralized hard tissues at a lower level of complexity. Bioinsp. Biomim. 2008, 3, 046003. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; He, J.; Sang, F.; Wang, Q.; Chen, L.; Cui, S.; Ding, B. Enhanced bone formation in electrospun poly(l-lactic-co-glycolic acid)–tussah silk fibroin ultrafine nanofiber scaffolds incorporated with graphene oxide. Mater. Sci. Eng. C 2016, 62, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Jung, H.; Byun, H. Preparation of Polyvinylidene fluoride nanofiber membrane and its antibacterial characteristics with nanosilver or graphene oxide. J. Nanosci. Nanotechnol. 2013, 13, 6269–6274. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, J.; Liao, C.Z.; Yeung, K.W.K.; Tjong, S.C. Novel electrospun polyvinylidene fluoride-graphene oxide-silver nanocomposite membranes with protein and bacterial antifouling characteristics. Express Polym Lett. 2018, 12, 365. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.H.; Kim, C.S. Electrospun bioactive poly(ε-caprolactone)–cellulose acetate–dextran antibacterial composite mats for wound dressing applications. Colloids Surf. A 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoo, H.S. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: In vitro and in vivo evaluation. J. Controlled Release 2010, 145, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Duque Sánchez, L.; Brack, N.; Postma, A.; Pigram, P.J.; Meagher, L. Surface modification of electrospun fibres for biomedical applications: A focus on radical polymerization methods. Biomaterials 2016, 106, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Wang, X.; Li, X.Y.; Chian, W.; Li, Y.; Liao, Y.Z. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers. Acta Biomater. 2013, 9, 5665–5672. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yang, H.; Zhen, S.J.; Huang, C.Z. Hydrogen-bond-mediated in situ fabrication of AgNPs/Agar/PAN electrospun nanofibers as reproducible sers substrates. ACS Appl. Mater. Interfaces 2015, 7, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Advanced materialsnanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Ke, P.; Jiao, X.N.; Ge, X.H.; Xiao, W.M.; Yu, B. From macro to micro: Structural biomimetic materials by electrospinning. RSC Adv. 2014, 4, 39704. [Google Scholar] [CrossRef]
- Viswanadam, G.; Chase, G.G. Modified electric fields to control the direction of electrospinning jets. Polymer 2013, 54, 1397–1404. [Google Scholar] [CrossRef]
- Forward, K.M.; Rutledge, G.C. Free surface electrospinning from a wire electrode. Chem. Eng. J. 2012, 183, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Huang, L.L.; Liu, F.; Faraz, N.; Han, L. Effect of flow rate on morphology and diameter of electrospun nanoporous microspheres. Adv. Sci. Lett. 2012, 10, 655–657. [Google Scholar] [CrossRef]
- Xie, J.W.; Liu, W.Y.; MacEwan, M.R.; Bridgman, P.C.; Xia, Y.N. Neurite outgrowth on electrospun nanofibers with uniaxial alignment: The effects of fiber density, surface coating, and supporting substrate. ACS Nano 2014, 8, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Sencadas, V.; Correia, D.M.; Areias, A.; Botelho, G.; Fonseca, A.M.; Neves, I.C.; Gomez Ribelles, J.L.; Lanceros Mendez, S. Determination of the parameters affecting electrospun chitosan fiber size distribution and morphology. Carbohydr. Polym. 2012, 87, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Pant, H.R.; Nam, K.T.; Oh, H.J.; Panthi, G.; Kim, H.D.; Kim, B.I.; Kim, H.Y. Effect of polymer molecular weight on the fiber morphology of electrospun mats. J. Colloid Interface Sci. 2011, 364, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Nasef, M.M.; Abbasi, A.; Majidi, R.F.; Takeshi, M. The Effect of Solution Viscosity and Concentration on Morphological Properties of Electrospun Nylon-6, 6 Nanofibers. 2012. Available online: http://epublication.cheme.utm.my/294/ (accessed on 21 August 2018).
- Tiwari, S.K.; Venkatraman, S.S. Importance of viscosity parameters in electrospinning: Of monolithic and core–shell fibers. Mater. Sci. Eng. C 2012, 32, 1037–1042. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.W.; Wong, S.C.; Abtahi, M.; Chen, P. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- He, J.H. Effect of temperature on surface tension of a bubble and hierarchical ruptured bubbles for nanofiber fabrication. Thermal Sci. 2012, 16, 327–330. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Tseng, H.F.; Chiu, Y.J.; Chen, J.T. Morphology transformations of electrospun polymer fibers annealed on polymer films with thickness-controlled growth rates of undulation. Polymer 2018, 134, 181–186. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of gelatin with tunable fiber morphology from round to flat/ribbon. Mater. Sci. Eng. C 2017, 80, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Pan, H.; Li, L.; Hu, L.; Cui, X. Continuous aligned polymer fibers produced by a modified electrospinning method. Polymer 2006, 47, 4901–4904. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Kotaki, M.; Mo, X.M.; Ramakrishna, S. Porous tubular structures with controlled fibre orientation using a modified electrospinning method. Nanotechnology 2005, 16, 918–924. [Google Scholar] [CrossRef]
- Fennessey, S.F.; Farris, R.J. Fabrication of aligned and molecularly oriented electrospun polyacrylonitrile nanofibers and the mechanical behavior of their twisted yarns. Polymer 2004, 45, 4217–4225. [Google Scholar] [CrossRef]
- Katta, P.; Alessandro, M.; Ramsier, R.D.; Chase, G.G. Continuous electrospinning of aligned polymer nanofibers onto a wire drum collector. Nano Lett. 2004, 4, 2215–2218. [Google Scholar] [CrossRef]
- Kameoka, J.; Orth, R.; Yang, Y.; Czaplewski, D.; Mathers, R.; Coates, G.W.; Craighead, H.G. A scanning tip electrospinning source for deposition of oriented nanofibres. Nanotechnology 2003, 14, 1124–1129. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.L.; Xia, Y.N. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Kriebel, A.; Rumman, M.; Scheld, M.; Hodde, D.; Brook, G.; Mey, J. Three-dimensional configuration of orientated fibers as guidance structures for cell migration and axonal growth. J. Biomed. Mater. Res. Part B 2014, 102, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Butcher, J.T. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kongkhlang, T.; Tashiro, K.; Kotaki, M.; Chirachanchai, S. Electrospinning as a new technique to control the crystal morphology and molecular orientation of polyoxymethylene nanofibers. J. Am. Chem. Soc. 2008, 130, 15460–15466. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, Y. Advanced multi-component nanostructures designed by dynamic shadowing growth. Nanoscale 2011, 3, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.L.; Hill, R.M.; Lowery, J.L.; Fridrikh, S.V.; Rutledge, G.C. Electrospun poly(styrene-block-dimethylsiloxane) block copolymer fibers exhibiting superhydrophobicity. Langmuir 2005, 21, 5549–5554. [Google Scholar] [CrossRef] [PubMed]
- Sas, I.; Gorga, R.E.; Joines, J.A.; Thoney, K.A. Literature review on superhydrophobic self-cleaning surfaces produced by electrospinning. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 824–845. [Google Scholar] [CrossRef] [Green Version]
- Fortin, N.; Klok, H.A. Glucose monitoring using a polymer brush modified polypropylene hollow fiber-based hydraulic flow sensor. ACS Appl. Mater. Interfaces 2015, 7, 4631–4640. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Ge, C.; Richardson, K.; Palmer, A.; Viapiano, M.; Lannutti, J.J. Microscale sensing of oxygen via encapsulated porphyrin nanofibers: Effect of indicator and polymer “core” permeability. ACS Appl. Mater. Interfaces 2015, 7, 8606–8614. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Li, X.Y.; Wang, X.; Yang, J.H.; Bligh, S.W.A.; Williams, G.R. Nanofibers fabricated using triaxial electrospinning as zero order drug delivery systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.P.; Lai, D.; Lounds, M.; Chung, K.; Kim, J.; Mansfield, J.F.; Takayama, S. Elongation of fibers from highly viscous dextran solutions enables fabrication of rapidly dissolving drug carrying fabrics. Adv. Healthcare Mater. 2015, 4, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.J.; Tseng, H.F.; Lo, Y.C.; Wu, B.H.; Chen, J.T. From electrospun polymer core–shell fibers to polymer hemispheres and spheres: Two types of transformation processes and tearing films with linearly arranged cavities. Macromolecules 2017, 50, 9024–9031. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Zhu, K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J. Controlled Release 2014, 193, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zou, T.; Li, S.; Jing, J.; Xia, X.; Liu, X. Drug-loaded zein nanofibers prepared using a modified coaxial electrospinning process. AAPS PharmSciTech 2013, 14, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, W.D.; Selomulya, C.; Chen, X.D. Facile spray-drying assembly of uniform microencapsulates with tunable core–shell structures and controlled release properties. Langmuir 2011, 27, 12910–12915. [Google Scholar] [CrossRef] [PubMed]
- Neubert, S.; Pliszka, D.; Thavasi, V.; Wintermantel, E.; Ramakrishna, S. Conductive electrospun PANi-PEO/TiO2 fibrous membrane for photo catalysis. Mater. Sci. Eng. B 2011, 176, 640–646. [Google Scholar] [CrossRef]
- Sun, B.; Duan, B.; Yuan, X. Preparation of core/shell PVP/PLA ultrafine fibers by coaxial electrospinning. J. Appl. Polym. Sci. 2006, 102, 39–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Jiang, L. Bio-mimic multichannel microtubes by a facile method. J. Am. Chem. Soc. 2007, 129, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, N.; Zhao, Y.; Jiang, L. Electrospinning of multilevel structured functional micro-/nanofibers and their applications. J. Mater. Chem. A 2013, 1, 7290–7305. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Zhang, X.; Lai, Y.; Wang, X.; Li, J.; Wei, G.; Su, Z. Electrospun doping of carbon nanotubes and platinum nanoparticles into the beta-phase polyvinylidene difluoride nanofibrous membrane for biosensor and catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Goor, O.J.G.M.; Hendrikse, S.I.S.; Dankers, P.Y.W.; Meijer, E.W. From supramolecular polymers to multi-component biomaterials. Chem. Soc. Rev. 2017, 46, 6621–6637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidoaki, S.; Kwon, I.K.; Matsuda, T. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials 2005, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Madduri, S.; Milleret, V.; Sulser, T.; Gobet, R.; Eberli, D. A bilayered hybrid microfibrous PLGA–acellular matrix scaffold for hollow organ tissue engineering. Biomaterials 2013, 34, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Layman, J.M.; Kenawy, E.R.; Watkins, J.R.; Carr, M.E.; Bowlin, G.L.; Wnek, G. Development of the Biohemostat—A Treatment Modality for High Pressure Bleeding Based on Super-Absorbent Polymers and Electrospun Membranes, Abstracts of Papers of The American Chemical Society, 2003. Amer Chemical Soc 1155 16th St, NW, Washington, DC 20036 USA: U436. Available online: http://oasys2.confex.com/acs/226nm/techprogram/P667059.HTM (accessed on 21 August 2018).
- Yang, Y.; Chen, Q.; Hsieh, Y.T.; Song, T.B.; De Marco, N.; Zhou, H.; Yang, Y. Multilayer transparent top electrode for solution processed Perovskite/Cu(In,Ga) (Se,S)(2) Four terminal tandem solar cells. ACS Nano 2015, 9, 7714–7721. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, Y.; Xin, C.; Wei, X.; Li, Q.; Lu, C.; Juang, Y.J. Dual electrode mode electrospinning of biodegradable polymers. Appl. Phys. Lett. 2008, 92, 213114. [Google Scholar] [CrossRef]
- Han, F.; Jia, X.; Dai, D.; Yang, X.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials 2013, 34, 7302–7313. [Google Scholar] [CrossRef] [PubMed]
- Vaseashta, A. Controlled formation of multiple taylor cones in electrospinning process. Appl. Phys. Lett. 2007, 90, 093115. [Google Scholar] [CrossRef]
- Dosunmu, O.O.; Chase, G.G.; Kataphinan, W.; Reneker, D.H. Electrospinning of polymer nanofibres from multiple jets on a porous tubular surface. Nanotechnology 2006, 17, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.K.; Shin, M.K.; Sohn, K.W.; Kim, S.I.; Kim, S.J.; Kim, S.K.; Lee, H.; Park, J.S. Direct fabrication of twisted nanofibers by electrospinning. Appl. Phys. Lett. 2007, 90, 263902. [Google Scholar] [CrossRef]
- Canejo, J.P.; Borges, J.P.; Godinho, M.H.; Brogueira, P.; Teixeira, P.I.C.; Terentjev, E.M. Helical twisting of electrospun liquid crystalline cellulose micro- and nanofibers. Adv. Mater. 2008, 20, 4821–4825. [Google Scholar] [CrossRef]
- Smit, E.; Bűttner, U.; Sanderson, R.D. Continuous yarns from electrospun fibers. Polymer 2005, 46, 2419–2423. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Latifi, M.; Teo, W.E.; Amani Tehran, M.; Ramakrishna, S. Producing continuous twisted yarn from well-aligned nanofibers by water vortex. Polym. Eng. Sci. 2011, 51, 323–329. [Google Scholar] [CrossRef]
- Dabirian, F.; Ravandi, S.A.H.; Sanatgar, R.H.; Hinestroza, J.P. Manufacturing of twisted continuous PAN nanofiber yarn by electrospinning process. Fibers Polym. 2011, 12, 610. [Google Scholar] [CrossRef]
- Teo, W.E.; Inai, R.; Ramakrishna, S. Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mater. 2011, 12, 013002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Chen, X.; Jing, X.; Fan, H.; Gu, Z.; Zhang, X. Fabrication and drug delivery of ultrathin mesoporous bioactive glass hollow fibers. Adv. Funct. Mater. 2010, 20, 1503–1510. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Chatterton, N.P.; Nazir, T.; Yu, D.G.; Zhu, L.M.; Branford-White, C.J. Electrospun nanofibers in drug delivery: Recent developments and perspectives. Ther. Delivery 2012, 3, 515–533. [Google Scholar] [CrossRef]
- Sahay, R.; Thavasi, V.; Ramakrishna, S. Design modifications in electrospinning setup for advanced applications. J Nanomater. 2011, 2011, 17. [Google Scholar] [CrossRef]
- Wunner, F.M.; Bas, O.; Saidy, N.T.; Dalton, P.D.; Pardo, E.M.D.J.; Hutmacher, D.W. Melt electrospinning writing of three-dimensional poly(ε-caprolactone) scaffolds with controllable morphologies for tissue engineering applications. JoVE 2017, 130, e56289. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Klinkhammer, K.; Salber, J.; Klee, D.; Möller, M. Direct in vitro electrospinning with polymer melts. Biomacromolecules 2006, 7, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, H.; Liu, Z.; Zhang, Q.; Fu, Q. Toward high-performance poly(L-lactide) fibers via tailoring crystallization with the aid of fibrillar nucleating agent. ACS Sustain. Chem. Eng. 2016, 4, 3939–3947. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Bertlein, S.; Hikimoto, D.; Hochleitner, G.; Hümmer, J.; Jungst, T.; Matsusaki, M.; Akashi, M.; Groll, J. Development of endothelial cell networks in 3d tissues by combination of melt electrospinning writing with cell-accumulation technology. Small 2018, 14, 1701521. [Google Scholar] [CrossRef] [PubMed]
- Muerza Cascante, M.L.; Shokoohmand, A.; Khosrotehrani, K.; Haylock, D.; Dalton, P.D.; Hutmacher, D.W.; Loessner, D. Endosteal-like extracellular matrix expression on melt electrospun written scaffolds. Acta Biomater. 2017, 52, 145–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.; Lemstra, P.J.; Kalb, B.; Pennings, A.J. Ultrahigh-strength polyethylene filaments by solution spinning and hot drawing. Polym. Bull. 1979, 1, 733–736. [Google Scholar] [CrossRef]
- Hoffmann, P.; Dutoit, B.; Salathé, R.P. Comparison of mechanically drawn and protection layer chemically etched optical fiber tips. Ultramicroscopy 1995, 61, 165–170. [Google Scholar] [CrossRef]
- Liu, S.H.; Liu, M.; Xu, Z.L.; Wei, Y.M.; Guo, X. A novel PES-TiO2 hollow fiber hybrid membrane prepared via sol-gel process assisted reverse thermally induced phase separation (RTIPS) method. J. Membr. Sci. 2017, 528, 303–315. [Google Scholar] [CrossRef]
- Canning, J.; Lindoy, L.; Huyang, G.; Naqshbandi, M.; Cook, K.; Crossley, M.J.; Luo, Y.; Peng, G.D.; Glavind, L.; Kristensen, M. Exploring the room temperature self-assembly of silica nanoparticle layers on optical fibres. SPIE 2013, 8793, 6. [Google Scholar]
- Tai, M.H.; Gao, P.; Tan, B.Y.L.; Sun, D.D.; Leckie, J.O. Highly efficient and flexible electrospun carbon–silica nanofibrous membrane for ultrafast gravity-driven oil–water separation. ACS Appl. Mater. Interfaces 2014, 6, 9393–9401. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Tan, J.Z.Y.; Kong, D.; Xiang, Q.; Wang, W.; Zhan, L.; Zeng, J.; Zhang, X. Electrospun Composite Nanofibers in Photoenergy Applications. Curr. Org. Chem. 2013, 17, 1382–1389. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Venugopal, J.R.; Ravichandran, R.; Ramakrishna, S. Electrospun inorganic and polymer composite nanofibers for biomedical applications AU - Sridhar, Radhakrishnan. J. Biomater. Sci. Polym. Ed. 2013, 24, 365–385. [Google Scholar]
- Hsu, K.C.; Liao, J.D.; Yang, J.R.; Fu, Y.S. Cellulose acetate assisted synthesis and characterization of kesterite quaternary semiconductor Cu2ZnSnS4 mesoporous fibers by an electrospinning process. CrystEngComm 2013, 15, 4303–4308. [Google Scholar] [CrossRef]
- Guo, A.; Roso, M.; Modesti, M.; Liu, J.; Colombo, P. Hierarchically structured polymer-derived ceramic fibers by electrospinning and catalyst-assisted pyrolysis. J. Eur. Ceram. Soc. 2014, 34, 549–554. [Google Scholar] [CrossRef]
- Sun, Y.; Qu, J.; Guo, Q.; Song, J.; Wei, G.; Xi, X.; Hou, G.; Qi, T. Preparation of fine-grained silica-doped zirconia fibers by electrospinning. Ceram. Int. 2017, 43, 12551–12556. [Google Scholar] [CrossRef]
- Lin, Y.P.; Lin, S.Y.; Lee, Y.C.; Chen-Yang, Y.W. High surface area electrospun prickle-like hierarchical anatase TiO2 nanofibers for dye-sensitized solar cell photoanodes. J. Mater. Chem. A 2013, 1, 9875–9884. [Google Scholar] [CrossRef]
- Sabba, D.; Mathews, N.; Chua, J.; Pramana, S.S.; Mulmudi, H.K.; Wang, Q.; Mhaisalkar, S.G. High-surface-area, interconnected, nanofibrillar TiO2 structures as photoanodes in dye-sensitized solar cells. Scr. Mater. 2013, 68, 487–490. [Google Scholar] [CrossRef]
- An, A.K.; Guo, J.; Lee, E.J.; Jeong, S.; Zhao, Y.; Wang, Z.; Leiknes, T. PDMS/PVDF hybrid electrospun membrane with superhydrophobic property and drop impact dynamics for dyeing wastewater treatment using membrane distillation. J. Membr. Sci. 2017, 525, 57–67. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, B.; Xu, J.; Xie, E.; Wang, T.; Xu, Z. Electrospinning–thermal treatment synthesis: A general strategy to decorate highly porous nanotubes on both internal and external side-walls with metal oxide/noble metal nanoparticles. Nanoscale 2013, 5, 2835–2839. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Poologasundarampillai, G.; van den Bergh, W.; Chater, R.J.; Kasuga, T.; Jones, J.R.; McPhail, D.S. Strategies for the chemical analysis of highly porous bone scaffolds using secondary ion mass spectrometry. Biomed. Mater. 2014, 9, 015013. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Smith, L.A.; Ma, P.X. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B 2004, 39, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Pedicini, A.; Farris, R.J. Mechanical behavior of electrospun polyurethane. Polymer 2003, 44, 6857–6862. [Google Scholar] [CrossRef]
- Kijeńska, E.; Prabhakaran, M.P.; Swieszkowski, W.; Kurzydlowski, K.J.; Ramakrishna, S. Interaction of schwann cells with laminin encapsulated PLCL core–shell nanofibers for nerve tissue engineering. Eur. Polym. J. 2014, 50, 30–38. [Google Scholar] [CrossRef]
- Xie, J.; Mao, H.; Yu, D.G.; Williams, G.R.; Jin, M. Highly stable coated polyvinylpyrrolidone nanofibers prepared using modified coaxial electrospinning. Fibers Polym. 2014, 15, 78–83. [Google Scholar] [CrossRef]
- Coaxial electrospinning of P (LLA-CL)/heparin biodegradable polymer nanofibers: Potential vascular graft for substitution of femoral artery. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jbm.b.32972 (accessed on 21 August 2018).
- Xu, W.; Ding, J.; Xiao, C.; Li, L.; Zhuang, X.; Chen, X. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials 2015, 54, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Controlled Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Livingston Arinzeh, T. Electrospun nanofibrous materials for neural tissue engineering. Polymers 2011, 3, 413–426. [Google Scholar] [CrossRef]
- Su, Y.; Su, Q.; Liu, W.; Lim, M.; Venugopal, J.R.; Mo, X.; Ramakrishna, S.; Al Deyab, S.S.; El Newehy, M. Controlled release of bone morphogenetic protein 2 and dexamethasone loaded in core–shell PLLACL–collagen fibers for use in bone tissue engineering. Acta Biomater. 2012, 8, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, S.; Wen, S.; Shen, M.; Zhu, M.; Shi, X. Characterization and antibacterial activity of amoxicillin-loaded electrospun nano-hydroxyapatite/poly(lactic-co-glycolic acid) composite nanofibers. Biomaterials 2013, 34, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.J.; Edirisinghe, M. Core-liquid-induced transition from coaxial electrospray to electrospinning of low-viscosity poly(lactide-co-glycolide) sheath solution. Macromolecules 2014, 47, 7930–7938. [Google Scholar] [CrossRef]
- Yu, D.G.; Chian, W.; Wang, X.; Li, X.Y.; Li, Y.; Liao, Y.Z. Linear drug release membrane prepared by a modified coaxial electrospinning process. J. Membr. Sci. 2013, 428, 150–156. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, X.; Han, F.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 2013, 34, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Han, F.; Wang, M.; Yuan, X. Controlled release of bovine serum albumin from electrospun fibrous membranes via an improved emulsion-core technique. J. Controlled Release 2011, 152, e181–e182. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Hu, P.; Xu, J.; Wang, A. Encapsulation of drug reservoirs in fibers by emulsion electrospinning: Morphology characterization and preliminary release assessment. Biomacromolecules 2006, 7, 2327–2330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Yang, D.Z.; Xu, F.; Zhang, Z.P.; Yin, R.X.; Nie, J. Electrospun core–shell structure nanofibers from homogeneous solution of poly(ethylene oxide)/chitosan. Macromolecules 2009, 42, 5278–5284. [Google Scholar] [CrossRef]
- Hu, J.; Tian, L.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Fabrication of nerve growth factor encapsulated aligned poly(ε-caprolactone) nanofibers and their assessment as a potential neural tissue engineering scaffold. Polymers 2016, 8, 54. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th Anniversary article: Galvanic replacement: A simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.B.; Cui, Y.; Yan, X.H.; Qi, W.; Yang, Y.; Wang, K.W.; He, Q.; Li, J.B. Controlled preparation of mno2 hierarchical hollow nanostructures and their application in water treatment. Adv. Mater. 2008, 20, 452–456. [Google Scholar] [CrossRef]
- Cao, S.W.; Zhu, Y.J.; Ma, M.Y.; Li, L.; Zhang, L. Hierarchically nanostructured magnetic hollow spheres of Fe3O4 and γ-Fe2O3: Preparation and potential application in drug delivery. J. Phys. Chem. C 2008, 112, 1851–1856. [Google Scholar] [CrossRef]
- Shen, Q.; Jiang, J.; Fan, M.; Liu, S.; Wang, L.; Fan, Q.; Huang, W. Prussian blue hollow nanostructures: Sacrificial template synthesis and application in hydrogen peroxide sensing. J. Electroanal. Chem. 2014, 712, 132–138. [Google Scholar] [CrossRef]
- Liu, S.W.; Huang, G.C.; Yu, J.G.; Ng, T.W.; Yip, H.Y.; Wongt, P.K. Porous fluorinated sno2 hollow nanospheres: Transformative self-assembly and photocatalytic inactivation of bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Limkrailassiri, K.; Gao, Y.; Chang, C.; Lin, L. Chip-to-chip fluidic connectors via near-field electrospinning, Micro Electro Mechanical Systems. Available online: https://ieeexplore.ieee.org/abstract/document/4433013 (accessed on 21 August 2018).
- Ma, M.; Krikorian, V.; Yu, J.H.; Thomas, E.L.; Rutledge, G.C. Electrospun polymer nanofibers with internal periodic structure obtained by microphase separation of cylindrically confined block copolymers. Nano Lett. 2006, 6, 2969–2972. [Google Scholar] [CrossRef] [PubMed]
- Tenne, R.; Homyonfer, M.; Feldman, Y. Nanoparticles of layered compounds with hollow cage structures (inorganic fullerene-like structures). Chem. Mater. 1998, 10, 3225–3238. [Google Scholar] [CrossRef]
- McCann, J.T.; Li, D.; Xia, Y. Electrospinning of nanofibers with core-sheath, hollow, or porous structures. J. Mater. Chem. 2005, 15, 735–738. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y.N. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Cheng, H.M.; Wei, Y.L.; Su, G.; Shen, Z.H. The influence of preparation parameters on the mass production of vapor-grown carbon nanofibers. Carbon 2000, 38, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.; Zhu, X.; Warren, R.; Wang, X.; He, T.; Lin, L.; Shen, J. Electrospinning of micro spiral fibers. Mater. Res. Express 2014, 1, 015302. [Google Scholar] [CrossRef]
- Lee, G.H.; Song, J.C.; Yoon, K.B. Controlled wall thickness and porosity of polymeric hollow nanofibers by coaxial electrospinning. Macromol. Res. 2010, 18, 571–576. [Google Scholar] [CrossRef]
- Li, D.; McCann, J.T.; Xia, Y. Use of Electrospinning to directly fabricate hollow nanofibers with functionalized inner and outer surfaces. Small 2005, 1, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Aravindan, V.; Kumar, P.S.; Liu, H.; Sundaramurthy, J.; Ramakrishna, S.; Madhavi, S. Synthesis of TiO2 hollow nanofibers by co-axial electrospinning and its superior lithium storage capability in full-cell assembly with olivine phosphate. Nanoscale 2013, 5, 5973–5980. [Google Scholar] [CrossRef] [PubMed]
- Akhondi, E.; Zamani, F.; Law, A.W.K.; Krantz, W.B.; Fane, A.G.; Chew, J.W. Influence of backwashing on the pore size of hollow fiber ultrafiltration membranes. J. Membr. Sci. 2017, 521, 33–42. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, L.; Zhang, Y.; Yang, Y.; Deng, C.; Yang, Z.; Chen, Q.; Wang, P.; Zheng, L. Electrospinning of Fe/SiC hybrid fibers for highly efficient microwave absorption. ACS Appl. Mater. Interfaces 2017, 9, 7265–7271. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hu, J.; Han, Z.; Wang, Z.; Zheng, Z.; Langer, J.; Economy, J. Synthesis of porous carbon fibers with strong anion exchange functional groups. Chem. Commun. 2015, 51, 9853–9856. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Bai, J.; Wang, J.; Liang, H.; Li, C.; Sun, W.; Meng, Q. Fabricating series of controllable-porosity carbon nanofibers-based palladium nanoparticles catalyst with enhanced performances and reusability. J. Mol. Catal. A Chem. 2015, 400, 95–103. [Google Scholar] [CrossRef]
- Smith, S.A.; Williams, B.P.; Joo, Y.L. Effect of polymer and ceramic morphology on the material and electrochemical properties of electrospun PAN/polymer derived ceramic composite nanofiber membranes for lithium ion battery separators. J. Membr. Sci. 2017, 526, 315–322. [Google Scholar] [CrossRef]
- Abeykoon, N.C.; Bonso, J.S.; Ferraris, J.P. Supercapacitor performance of carbon nanofiber electrodes derived from immiscible PAN/PMMA polymer blends. RSC Adv. 2015, 5, 19865–19873. [Google Scholar] [CrossRef]
- Zhao, H.; Gu, W.; Thielke, M.; Sterner, E.; Tsai, T.; Russell, T.P.; Bryan Coughlin, E.; Theato, P. Functionalized nanoporous thin films and fibers from photocleavable block copolymers featuring activated esters. Macromolecules 2013, 46, 5195–5201. [Google Scholar] [CrossRef]
- Bognitzki, M.; Frese, T.; Steinhart, M.; Greiner, A.; Wendorff, J.H.; Schaper, A.; Hellwig, M. Preparation of fibers with nanoscaled morphologies: Electrospinning of polymer blends. Polym. Eng. Sci. 2001, 41, 982–989. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured fibers via electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Bae, H.S.; Haider, A.; Selim, K.M.K.; Kang, D.Y.; Kim, E.J.; Kang, I.K. Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine. J. Polym. Res. 2013, 20, 158. [Google Scholar] [CrossRef]
- Fashandi, H.; Karimi, M. Pore formation in polystyrene fiber by superimposing temperature and relative humidity of electrospinning atmosphere. Polymer 2012, 53, 5832–5849. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, X.; Xu, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Ultrafine fibers electrospun from biodegradable polymers. J. Appl. Polym. Sci. 2003, 89, 1085–1092. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, X.; Jing, X.; Fan, H.; Guo, B.; Gu, Z.; Zhang, X. Preparation, bioactivity, and drug release of hierarchical nanoporous bioactive glass ultrathin fibers. Adv. Mater. 2010, 22, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, Y.; Zhuang, X.; Chen, X.; Jing, X. Electrospinning of multicomponent ultrathin fibrous nonwovens for semi-occlusive wound dressings. J. Biomed. Mater. Res. Part A 2009, 89A, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Sun, Z.; Hong, Y.; Li, Y.; Chen, X.; Zhang, X. Reverse-biomineralization assembly of acid-sensitive biomimetic fibers for hard tissue engineering and drug delivery. J. Mater. Chem. B 2013, 1, 3694–3704. [Google Scholar] [CrossRef]
- He, G.; Mandlmeier, B.; Schuster, J.; Nazar, L.F.; Bein, T. Bimodal mesoporous carbon nanofibers with high porosity: Freestanding and embedded in membranes for lithium-sulfur batteries. Chem. Mater. 2014, 26, 3879–3886. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Tampau, A.; González Martínez, C.; Chiralt, A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocolloids 2018, 79, 158–169. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer structures based on annealed electrospun biopolymer coatings of interest in water and aroma barrier fiber-based food packaging applications. J. Appl. Polym. Sci. 2018, 135, 45501. [Google Scholar] [CrossRef]
- Yin, X.; Wen, Y.; Li, Y.; Liu, P.; Li, Z.; Shi, Y.; Lan, J.; Guo, R.; Tan, L. Facile fabrication of sandwich structural membrane with a hydrogel nanofibrous mat as inner layer for wound dressing application. Front. Chem. 2018, 6, 490. [Google Scholar] [CrossRef] [PubMed]
- Hassiba, A.J.; El Zowalaty, M.E.; Webster, T.J.; Abdullah, A.M.; Nasrallah, G.K.; Khalil, K.A.; Luyt, A.S.; Elzatahry, A.A. Synthesis, characterization, and antimicrobial properties of novel double layer nanocomposite electrospun fibers for wound dressing applications. Int. J. Nanomed. 2017, 12, 2205–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.W.; Lee, H.B.; Yeon, S.M.; Park, J.; Lee, H.J.; Yoon, J.; Park, S.H. Enhanced piezoelectricity in a robust and harmonious multilayer assembly of electrospun nanofiber mats and microbead-based electrodes. ACS Appl. Mater. Interfaces 2018, 10, 5723–5730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Yan, X.; He, H.W.; Liu, M.N.; Wang, X.X.; Nie, G.D.; Zhang, J.; Fu, J.; Long, Y.Z. Solvent-free two-component electrospinning of ultrafine polymer fibers. New J. Chem. 2018, 42, 11739–11745. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Yu, D.G.; Zhang, D.F.; Chatterton, N.P.; White, K.N. Structure-tunable Janus fibers fabricated using spinnerets with varying port angles. Chem. Commun. 2015, 51, 4623–4626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Yu, D.G.; Pan, D.; Liu, X.K.; Wang, X.; Bligh, S.W.A.; Williams, G.R. Electrospun pH-sensitive core–shell polymer nanocomposites fabricated using a tri-axial process. Acta Biomater. 2016, 35, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; He, H.; Zhang, X.; Yan, X.; Li, J.; Chen, F.; Yuan, D.; Ning, X. Efficient synthesis of PVDF/PI side-by-side bicomponent nanofiber membrane with enhanced mechanical strength and good thermal stability. Nanomaterials 2018, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Wilkes, G.L. Some investigations on the fiber formation by utilizing a side-by-side bicomponent electrospinning approach. Polymer 2003, 44, 6353–6359. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, J.J.; Zhang, M.; Williams, G.R. High-quality Janus nanofibers prepared using three-fluid electrospinning. Chem. Commun. 2017, 53, 4542–4545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Jiang, S.; Seuß, M.; Fery, A.; Lang, G.; Scheibel, T.; Agarwal, S. Two-in-one composite fibers with side-by-side arrangement of silk fibroin and poly(L-lactide) by electrospinning. Macromol. Mater. Eng. 2016, 301, 48–55. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, P.; Wang, Q.; Liu, Y.; Pan, K. Novel PAN/PVP Janus ultrafine fiber membrane and its application for biphasic drug release. J. Mater. Chem. B 2017, 5, 5390–5396. [Google Scholar] [CrossRef]
- Yu, D.G.; Yang, C.; Jin, M.; Williams, G.R.; Zou, H.; Wang, X.; Annie Bligh, S.W. Medicated janus fibers fabricated using a teflon-coated side-by-side spinneret. Colloids Surf. B 2016, 138, 110–116. [Google Scholar] [CrossRef] [PubMed]









| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Phase separation | High porosity | Thin fibers and small pores | [5,6] |
| Template synthesis | Designed fiber morphology | Low porosity | [24] |
| Melt-blown | High efficiency, commercial | Instability, fiber diameter exceeding 1–2 μm | [8,9] |
| Self-assembly | A simple route to synthesize multifunctional nanofibers | Introduction of the organic solvent | [10,11] |
| 3D printing | Controlled pore size | Low porosity | [25] |
| Electrospinning | Easy process and controlled fiber morphology | Small pores | [14,15,16] |
| Composition | Solvent | Concentration | Functionality and Applications | References |
|---|---|---|---|---|
| Polymetylmethacrylate (PMMA) | Tetrahydrofuran (THF), acetone, chloroform | 10 wt% | Superhydrophobic units for active packaging | [44] |
| Polyvinyl alcohol (PVA) | DI water | 8–16 wt% and 1–10 wt% | Biofilters and biomembranes | [45,46] |
| Poly (lactic-co-glycolic acid) PLGA | Polysorbate 80, ethanol/ethyl acetate | 4 wt% | Produced by a low-energy nano-emulsification approach, an easily scalable methodology, appropriate for the pharmaceutical industries | [47] |
| Polycaprolactone (PCL) | Chloroform and acetone | 10% (W/W) | Show great potential for further formulation as oromucosal drug delivery systems | [48,49] |
| Poly (L-lactic acid) (PLLA) | N,N-dimethyl-formamide (DMF) and dichloromethane (MC) | 10 wt% | Sterilize PLLA membranes for clinical applications in regenerative medicine | [50] |
| Gelatin | DI water | 30–50% (W/V) | For tissue regeneration, the versatility of this biomaterial | [51] |
| Chitosan | Trifluoroacetic acid (TFA) | 1–6 wt% | Tissue engineering properties and wound healing | [52,53] |
| Starch | Dimethyl sulfoxide (DMSO), glutaraldehyde | 25 wt% | Applications in the fields of tissue engineering, pharmaceutical therapy, and medical | [54] |
| Collagen | TFA | 42.85% (W/W) | Supports cell attachment and growth, form fibrous tissue engineering scaffolds | [55] |
| PLGA-curcumin | Chloroform/methanol | 40 wt%/60 wt% | Delivering curcumin over a long period in a controlled manner | [56,57] |
| PLGA–collagen | Hexafluoroiso-propanol (HFIP) | 20% (W/V) | For bioengineered skin substitutes | [58] |
| PCL–chitosan | HFIP and acetic acid | 20:1 (W/W) | The fast degradation profile leads to rapid cellular infiltration, improved vascular remodeling, and neotissue formation without calcification or aneurysm | [59] |
| Poly(ε-hydroxybutyrate-co-ε-hydroxyvalerate) PHBV–gelatin | Tetrafluoro-ethylene (TFE) | 50 wt% | Serves as a useful alternative carrier for ocular surface tissue engineering and use as an alternative substrate to amniotic membrane | [60,61] |
| Hydroxyapatite (HAP)–tussah silk fibroin | Ammonia, citric acid | 31 wt% | Supply as scaffolds in tissue engineering and bone regeneration | [62] |
| Poly(lactic acid) (PLA)/PCL–cellulose nanocrystals | Acetone, DCM, toluene with phosphorus pentoxide | 1wt% | Biodegradable character, use in different areas such as biomedicine or food packaging | [63] |
| PVA/alginate-bioglass | DI water | 10 wt% | With proper biological and mechanical properties for soft/hard tissue applications | [64,65] |
| Polycatecholamine/CaCO3-collagen | HFIP, CaCl2 solution | 8% (W/V), 10% (W/W) | Provide multifunctional scaffold properties for possible bone tissue engineering applications | [66] |
| PCL/(polyvinylpyrrolidone) PVP-trans-anethole | Chloroform: methanol | 10% (W/V), 30% (W/V) | Promoting in vitro osteoblast differentiation, we can help with site-specific repair and regeneration of bone tissue | [67] |
| Polyurethane (PU)–dextran–estradiol | DMSO and THF | 10 wt% | Post-menopausal wound dressing | [68] |
| PVA–PVP–HAP | DMSO | 2.5, 5, 8.5, 10, and 15 wt% | Sensor, anti-static, microwave absorbing, and conductive coating | [69,70] |
| PLGA–tussah silk–graphene oxide | HFIP | 13 wt% | Cancer treatment, therapeutic patch for drug delivery, and an excellent scaffold material for bone tissue engineering | [71] |
| Polyvinylidene fluoride (PVDF)–graphene oxide–silver | Acetone and DMF | 2 wt% | Micro and nanoscale magnetoelectric devices, magnetic-field sensors, and energy-harvesters | [72,73] |
| Poly (ε-caprolactone)–cellulose acetate–dextran–tetracycline hydrochloride | DMF, THF | 10 wt% | Good bioactivity, high cell attachment and proliferation, effective antibiotic activity against bacteria, for wound dressing and skin engineering applications | [74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules 2019, 24, 834. https://doi.org/10.3390/molecules24050834
Wang C, Wang J, Zeng L, Qiao Z, Liu X, Liu H, Zhang J, Ding J. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules. 2019; 24(5):834. https://doi.org/10.3390/molecules24050834
Chicago/Turabian StyleWang, Chenyu, Jun Wang, Liangdan Zeng, Ziwen Qiao, Xiaochen Liu, He Liu, Jin Zhang, and Jianxun Ding. 2019. "Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies" Molecules 24, no. 5: 834. https://doi.org/10.3390/molecules24050834





