Fish Protein and Lipid Interactions on the Digestibility and Bioavailability of Starch and Protein from Durum Wheat Pasta
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Predictive Glycaemic Response
2.2. Protein Content and In Vitro Protein Digestibility
2.3. The Composition of Amino Acids Released into Intestine After In Vitro Digestion
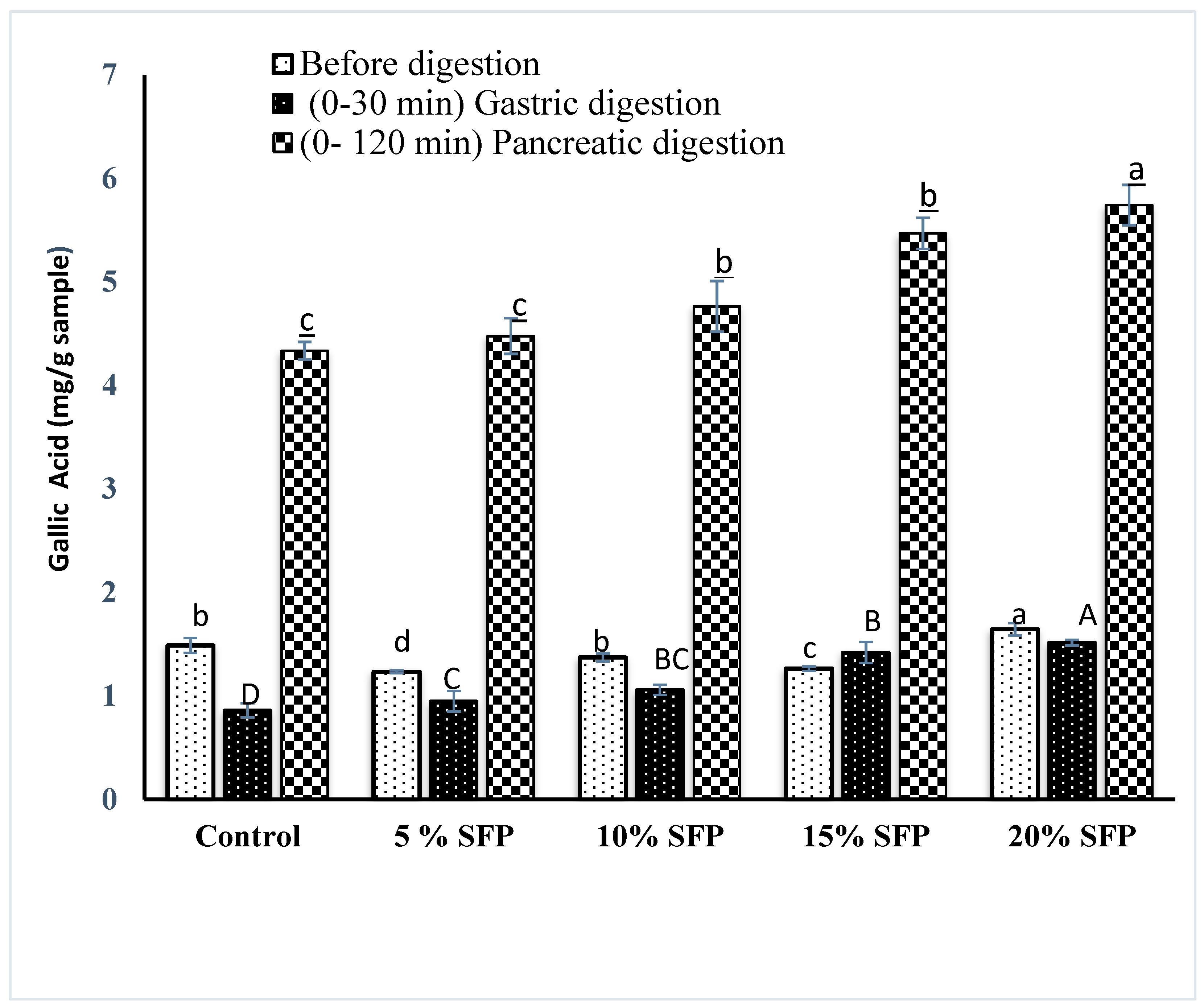
2.4. In vitro Bioaccessibility of Phenolic content and Antioxidant activity
3. Materials and Methods
3.1. Materials
3.2. Fish Powder Preparation
3.3. Pasta Preparation
3.4. In Vitro Starch Digestibility and Glycaemic Response
3.5. In Vitro Protein Digestibility of pasta
3.6. Amino Acid Profile
3.7. In Vitro Gastro-Intestinal Digestion
3.8. Total Phenolic Content and Antioxidant Activity of pasta
3.9. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.9.1. Chemical Reagents and Standard Solutions
3.9.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sae-leaw, T.; Benjakul, S. Antioxidant activities of hydrolysed collagen from salmon scale ossein prepared with the aid of ultrasound. Int. J. Food Sci. Technol. 2018, 53, 2786–2795. [Google Scholar] [CrossRef]
- Aubourg, S.P. Impact of high-pressure processing on chemical constituents and nutritional properties in aquatic foods: A review. Int. J. Food Sci. Technol. 2018, 53, 873–891. [Google Scholar] [CrossRef]
- Matos, J.; Lourenço, H.M.; Brito, P.; Maulvault, A.L.; Martins, L.L.; Afonso, C. Influence of bioaccessibility of total mercury, methyl-mercury and selenium on the risk/benefit associated to the consumption of raw and cooked blue shark (Prionace glauca). Environ. Res. 2015, 143, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.S.; Lourenco, L.F.H.; Sousa, C.L.; Peixoto Joele, M.R.S.; Ribeiro, S.C.A. Composition of MSM from Brazilian catfish and technological properties of fish flour. Food Control 2015, 50, 38–44. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Ready-to-eat snack products: The role of extrusion technology in developing consumer acceptable and nutritious snacks. Int. J. Food Sci. Technol. 2013, 48, 893–902. [Google Scholar] [CrossRef]
- Torres, A.; Frias, J.; Granito, M.; Vidal-Valverde, C. Germinated Cajanus cajan seeds as ingredients in pasta products: Chemical, biological and sensory evaluation. Food Chem. 2006, 101, 202–211. [Google Scholar] [CrossRef]
- Liu, T.; Hamid, N.; Kantono, K.; Pereira, L.; Farouk, M.M.; Knowles, S.O. Effects of meat addition on pasta structure, nutrition and in vitro digestibility. Food Chem. 2016, 213, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Tazrart, K.; Lamacchia, C.; Zaidi, F.; Haros, M. Nutrient composition and in vitro digestibility of fresh pasta enriched with Vicia faba. J. Food Compos. Anal. 2016, 47, 8–15. [Google Scholar] [CrossRef]
- Chen, X.; He, X.W.; Zhang, B.; Fu, X.; Jane, J.L.; Huang, Q. Effects of adding corn oil and soy protein to corn starch on the physicochemical and digestive properties of the starch. Int. J. Biol. Macromol. 2017, 104, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An international scientific consensus summit from the international carbohydrate quality consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. Synergistic effect of different dietary fibres in pasta on in vitro starch digestion? Food Chem. 2015, 172, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Oyeyinka, S.A.; Singh, S.; Venter, S.L.; Amonsou, E.O. Effect of lipid types on complexation and some physicochemical properties of bambara groundnut starch. Starch/Staerke 2017, 69, 1–10. [Google Scholar] [CrossRef]
- Lau, E.; Zhou, W.; Henry, C.J. Effect of fat type in baked bread on amylose–lipid complex formation and glycaemic response. Br. J. Nutr. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT-Food Sci. Technol. 2017, 82, 296–302. [Google Scholar] [CrossRef]
- Ramya, N.S.; Prabhasankar, P.; Gowda, L.R.; Modi, V.K.; Bhaskar, N. Influence of freeze-dried shrimp meat in pasta processing qualities of Indian T. durum Wheat. J. Aquat. Food Prod. Technol. 2014, 24, 582–596. [Google Scholar] [CrossRef]
- Vijaykrishnaraj, M.; Bharath Kumar, S.; Prabhasankar, P. Green mussel (Perna canaliculus) as a marine ingredient to enrich gluten free pasta: Product quality, microstructure and biofunctional evaluation. J. Food Meas. Charact. 2014, 9, 76–85. [Google Scholar] [CrossRef]
- Montalbano, A.; Tesoriere, L.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Parrino, B.; Attanzio, A.; Livrea, M.A.; Cascioferro, S.; et al. Quality characteristics and in vitro digestibility study of barley flour enriched ditalini pasta. LWT-Food Sci. Technol. 2016, 72, 223–228. [Google Scholar] [CrossRef]
- Cardenas-Hernandez, A.; Beta, T.; Loarca-Pica, G.; Castao-Tostado, E.; Nieto-Barrera, J.O.; Mendoza, S. Improved functional properties of pasta: Enrichment with amaranth seed flour and dried amaranth leaves. J. Cereal Sci. 2016, 72, 84–90. [Google Scholar] [CrossRef]
- Bouacida, S.; Ben Amira, A.; Ben Haj Koubaier, H.; Blecker, C.; Bouzouita, N. Chemical composition, cooking quality, texture and consumer acceptance of pasta with Eruca vesicaria leaves. Int. J. Food Sci. Technol. 2017, 52, 2248–2255. [Google Scholar] [CrossRef]
- Pasqualone, A.; Punzi, R.; Trani, A.; Summo, C.; Paradiso, V.M.; Caponio, F.; Gambacorta, G. Enrichment of fresh pasta with antioxidant extracts obtained from artichoke canning by-products by ultrasound-assisted technology and quality characterisation of the end product. Int. J. Food Sci. Technol. 2017, 1–10. [Google Scholar] [CrossRef]
- Martínez, M.L.; Marín, M.A.; Gili, R.D.; Penci, M.C.; Ribotta, P.D. Effect of defatted almond flour on cooking, chemical and sensorial properties of gluten-free fresh pasta. Int. J. Food Sci. Technol. 2017, 52, 2148–2155. [Google Scholar] [CrossRef]
- Rodríguez De Marco, E.; Steffolani, M.E.; Martínez, M.; León, A.E. The use of Nannochloropsis sp. as a source of omega-3 fatty acids in dry pasta: Chemical, technological and sensory evaluation. Int. J. Food Sci. Technol. 2018, 53, 499–507. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.; Molla, M.M.; Wang, C.; Diao, X.; Shen, Q. In vitro starch digestibility and in vivo glycemic response of foxtail millet and its products. Food Funct. 2016, 1, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Giuberti, G.; Gallo, A.; Cerioli, C.; Fortunati, P.; Masoero, F. Cooking quality and starch digestibility of gluten free pasta using new bean flour. Food Chem. 2015, 175, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Annor, G.A.; Marcone, M.; Corredig, M.; Bertoft, E.; Seetharaman, K. Effects of the amount and type of fatty acids present in millets on their in vitro starch digestibility and expected glycemic index (eGI). J. Cereal Sci. 2015, 64, 76–81. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Heiniö, R.L.; Cassan, D.; Holopainen-Mantila, U.; Micard, V.; Lantto, R.; Sozer, N. Effect of bioprocessing and fractionation on the structural, textural and sensory properties of gluten-free faba bean pasta. LWT-Food Sci. Technol. 2016, 67, 27–36. [Google Scholar] [CrossRef]
- Djeukeu, W.A.; Gouado, I.; Leng, M.S.; Vijaykrishnaraj, M.; Prabhasankar, P. Effect of dried yam flour (Dioscorea schimperiana) on cooking quality, digestibility profile and antioxidant potential of wheat based pasta. J. Food Meas. Charact. 2017, 11, 1421–1429. [Google Scholar] [CrossRef]
- Chillo, S.; Monro, J.A.; Mishra, S.; Henry, C.J. Effect of incorporating legume flour into semolina spaghetti on its cooking quality and glycaemic impact measured in vitro. Int. J. Food Sci. Nutr. 2010, 61, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, M.A.; Drago, S.R.; Bassett, M.N.; Lobo, M.O.; Samman, N.C. Nutritional improvement of corn pasta-like product with broad bean (Vicia faba) and quinoa (Chenopodium quinoa). Food Chem. 2016, 199, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Seczyk, L.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves-The influence of protein-phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Kadam, S.U.; Prabhasankar, P. Evaluation of cooking, microstructure, texture and sensory quality characteristics of shrimp meat-based pasta. J. Texture Stud. 2012, 43, 268–274. [Google Scholar] [CrossRef]
- Prodpran, T.; Benjakul, S.; Phatcharat, S. Effect of phenolic compounds on protein cross-linking and properties of film from fish myofibrillar protein. Int. J. Biol. Macromol. 2012, 51, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Cavada, E.; Drago, S.R.; González, R.J.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Effects of the addition of wild legumes (Lathyrus annuus and Lathyrus clymenum) on the physical and nutritional properties of extruded products based on whole corn and brown rice. Food Chem. 2011, 128, 961–967. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, M.; Huang, H. Changes of heat-treated soymilks in bioactive compounds and their antioxidant activities under in vitro gastrointestinal digestion. Eur. Food Res. Technol. 2014, 239, 637–652. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Turco, I.; Bacchetti, T.; Bender, C.; Zimmermann, B.; Oboh, G.; Ferretti, G. Polyphenol content and glycemic load of pasta enriched with Faba bean flour. Funct. Foods Health Dis. 2016, 6, 291–305. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyz, J. The influence of protein-flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Seczyk, L.; Swieca, M.; Gawlik-Dziki, U.; Luty, M.; Czyz, J. Effect of fortification with parsley (Petroselinum crispum Mill.) leaves on the nutraceutical and nutritional quality of wheat pasta. Food Chem. 2016, 190, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D.; Jin, D.; Waterhouse, G.I.N. Effect of adding elderberry juice concentrate on the quality attributes, polyphenol contents and antioxidant activity of three fibre-enriched pastas. Food Res. Int. 2013, 54, 781–789. [Google Scholar] [CrossRef]
- Gao, J.; Brennan, M.A.; Mason, S.L.; Brennan, C.S. Effect of sugar replacement with stevianna and inulin on the texture and predictive glycaemic response of muffins. Int. J. Food Sci. Technol. 2016, 51, 1979–1987. [Google Scholar] [CrossRef]
- Hsu, H.; Vavak, D. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 45, 1269–1273. [Google Scholar] [CrossRef]
- Heems, D.; Luck, G.; Fraudeau, C.; Verette, E. Fully automated precolumn derivatization, on-line dialysis and high-performance liquid chromatographic analysis of amino acids in food, beverages and feedstuff. J. Chromatogr. A 1998, 798, 9–17. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Evaluation of antioxidant activity and electronic taste and aroma properties of antho-beers from purple wheat grain. J. Agric. Food Chem. 2007, 55, 8958–8966. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors due to biosecurity regulations. |





| Samples | PC in Raw Pasta (g/100 g Dry Pasta) | PC in Cooked Pasta (g/100 g Dry Pasta) | PD (%) | PA (g/100 g Dry Pasta) |
|---|---|---|---|---|
| CP | 12.60 ± 0.05 a | 12.88 ± 0.06 a | 86.41 ± 0.37 a | 11.13± 0.07 a |
| SFP 5 | 14.34 ± 0.03 b | 15.41 ± 0.17 b | 84.60 ± 0.20 b | 13.03 ± 0.14 b |
| SFP 10 | 17.67 ± 0.04 c | 18.10 ± 0.11 c | 82.97 ± 0.10 c | 15.02 ± 0.09 c |
| SFP 15 | 20.73 ± 0.10 d | 20.77 ± 0.09 d | 81.16 ± 0.27 d | 16.85 ± 0.12 d |
| SFP 20 | 22.7 ± 0.30 e | 23.40 ± 0.13 e | 81.95 ± 0.18 e | 19.18 ± 0.07 e |
| Amino Acid | CP | SFP5 | SFP10 | SFP15 | SFP20 |
|---|---|---|---|---|---|
| Phenylalanine | 18.07 ± 0.17 a | 14.63 ± 1.13 bc | 14.86 ± 1.09 b | 13.40± 0.46 bc | 12.68 ± 0.31 c |
| Tyrosine | 14.03 ± 0.37 a | 12.07 ± 0.96 b | 12.61 ± 1.07 ab | 11.40 ± 0.30 b | 10.90 ± 0.39 b |
| Isoleucine | 14.94 ± 0.10 a | 12.55 ± 1.05 a | 13.48 ± 1.09 ab | 12.24 ± 0.31 b | 11.98 ± 0.23 b |
| Leucine | 26.82 ± 0.21 a | 22.72 ± 1.78 b | 23.92 ± 1.86 ab | 21.87 ± 0.60 b | 21.68 ± 0.29 b |
| Lysine | 16.15 ± 0.33 b | 16.72 ± 1.30 b | 21.58 ± 1.58 a | 21.41 ± 0.58 a | 22.27 ± 1.54 a |
| Methionine | 0.57 ± 0.35 a | 0.81 ± 0.61 a | 0.51 ± 0.17 a | 0.47 ± 0.15 a | 0.46 ± 0.08 a |
| Threonine | 13.14 ± 0.18 a | 11.67 ± 0.90 a | 13.18 ± 0.98 a | 12.08 ± 0.38 a | 11.96 ± 0.42 a |
| Tryptophan | 6.85 ± 0.84 a | 5.56 ± 0.96 a | 6.05 ± 0.24 a | 5.44 ± 0.44 a | 5.21 ± 0.07 a |
| Valine | 16.42 ± 0.55 a | 14.09 ± 1.44 a | 15.72 ± 1.28 a | 14.25 ± 0.31 a | 13.92 ± 0.58 a |
| ΣEAAs | 126.99 | 110.82 | 121.91 | 112.56 | 111.06 |
| NEAAs | |||||
| Argine | 21.64 ± 0.22 ab | 18.13 ± 1.58 b | 23.60 ± 1.38 a | 21.04 ± 1.37 ab | 20.44 ± 2.07 ab |
| Alanine | 14.32 ± 0.09 a | 13.05 ± 0.97 a | 14.63 ± 1.10 a | 13.71 ± 0.34 a | 13.75 ± 0.22 a |
| Glutamic acid | 85.31 ± 13.45 a | 73.76 ± 5.11 ab | 77.68 ± 5.86 ab | 65.44 ± 0.92 ab | 55.85 ± 4.59 b |
| Glycine | 17.00 ± 0.33 a | 14.61 ± 1.01 b | 15.02 ± 1.22 ab | 13.98 ± 0.58 b | 13.53 ± 0.54 b |
| Proline | 37.87 ± 2.08 a | 23.43 ± 4.20 b | 25.67 ± 2.20 b | 21.64 ± 0.72 b | 19.32 ± 1.90 b |
| Serine | 16.15 ± 1.32 a | 14.08 ± 1.21 ab | 15.00 ± 1.22 ab | 13.33 ± 0.33 ab | 12.43 ± 1.00 b |
| Asparagine | 20.58 ± 0.41 ab | 19.50 ± 1.50 b | 23.03 ± 1.78 a | 21.29± 0.70 ab | 21.19 ± 0.63 ab |
| ΣNEAAs | 212.87 | 176.56 | 194.63 | 170.43 | 156.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, A.S.; Brennan, M.A.; Guo, X.; Zeng, X.-A.; Brennan, C.S. Fish Protein and Lipid Interactions on the Digestibility and Bioavailability of Starch and Protein from Durum Wheat Pasta. Molecules 2019, 24, 839. https://doi.org/10.3390/molecules24050839
Desai AS, Brennan MA, Guo X, Zeng X-A, Brennan CS. Fish Protein and Lipid Interactions on the Digestibility and Bioavailability of Starch and Protein from Durum Wheat Pasta. Molecules. 2019; 24(5):839. https://doi.org/10.3390/molecules24050839
Chicago/Turabian StyleDesai, Ajay S., Margaret A. Brennan, Xinbo Guo, Xin-An Zeng, and Charles S. Brennan. 2019. "Fish Protein and Lipid Interactions on the Digestibility and Bioavailability of Starch and Protein from Durum Wheat Pasta" Molecules 24, no. 5: 839. https://doi.org/10.3390/molecules24050839
APA StyleDesai, A. S., Brennan, M. A., Guo, X., Zeng, X.-A., & Brennan, C. S. (2019). Fish Protein and Lipid Interactions on the Digestibility and Bioavailability of Starch and Protein from Durum Wheat Pasta. Molecules, 24(5), 839. https://doi.org/10.3390/molecules24050839








