In Vitro and In Vivo Anti-Inflammatory Effects of Polyphyllin VII through Downregulating MAPK and NF-κB Pathways
Abstract
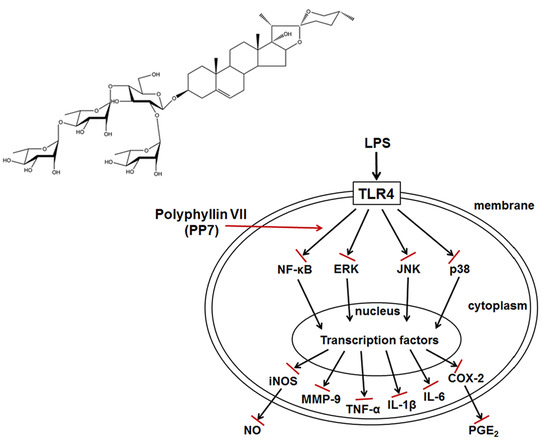
:1. Introduction
2. Results
2.1. Effects of PP7 on LPS-Induced NO and PGE2 Production in RAW264.7 Cells
2.2. Effects of PP7 on LPS-Induced Pro-Inflammatory Cytokines Protein and mRNA Expression in RAW264.7 Cells
2.3. Effects of PP7 on LPS-Induced NF-κB p65 Nuclear Translocation and IκB-α Phosphorylation and Degradation in RAW264.7 Cells
2.4. Effects of PP7 on LPS-Induced MAPKs Activation and MMP-9 Expression
2.5. Effect of PP7 on Xylene-Induced Ear Edema in Mice
2.6. Effect of PP7 on Cotton Pellet-Induced Granuloma in Mice
2.7. Anti-Inflammatory and Protective Effects of PP7 in LPS-Induced Inflammation in Zebrafish Embryo
2.8. PP7 Inhibits CuSO4-Induced Inflammation in Zebrafish
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Drug Treatments
4.3. Cell Viability Assay
4.4. Determination of NO, PGE2, TNF-α, IL-1β, and IL-6 Production
4.5. Preparation of Cytosolic and Nuclear Extracts for NF-κB Detection
4.6. Quantitative Reverse Transcription Polymerase Chain Reaction (qPCR) Analysis
4.7. Western Blotting
4.8. Animals
4.9. Xylene-Induced Ear Edema in Mice
4.10. Cotton Pellet-Induced Granuloma in Mice
4.11. Measurement of NO Production in Zebrafish
4.12. Neutrophil Migration Assay in Zebrafish
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruscia, E.M.; Bonfield, T.L. Cystic Fibrosis Lung Immunity: The Role of the Macrophage. J. Innate Immun. 2016, 8, 550–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decano, J.L.; Mattson, P.C.; Aikawa, M. Macrophages in Vascular Inflammation: Origins and Functions. Curr. Atheroscler. Rep. 2016, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.K.; Musa, M.; Samsudin, A.R.; Sosroseno, W. Role of interleukin-1beta and tumour necrosis factor-alpha on hydroxyapatite-induced phagocytosis by murine macrophages (RAW264.7 cells). Br. J. Biomed. Sci. 2006, 63, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S. Nitric oxide: Discovery and impact on clinical medicine. J. R. Soc. Med. 1999, 92, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Chen, L.C.; Gordon, M.A.; Laskin, J.D.; Laskin, D.L. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J. Leukoc. Biol. 2002, 71, 1005–1011. [Google Scholar] [PubMed]
- Bondeson, J. The mechanisms of action of disease-modifying antirheumatic drugs: A review with emphasis on macrophage signal transduction and the induction of proinflammatory cytokines. Gen. Pharmacol. 1997, 29, 127–150. [Google Scholar] [CrossRef]
- Matsuno, R.; Aramaki, Y.; Arima, H.; Adachi, Y.; Ohno, N.; Yadomae, T.; Tsuchiya, S. Contribution of CR3 to Nitric Oxide Production from Macrophages Stimulated with High-Dose of LPS. Biochem. Biophys. Res. Commun. 1998, 244, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.M.; Mark, H.; Watkins, L.R.; Hang, Y. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010, 114, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Huang, S.S.; Deng, J.S. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo. PLoS ONE 2012, 7, e35922. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Yang, C.M.; Chang, Y.S.; Amagaya, S.; Wang, H.C.; Hou, W.C.; Huang, S.S.; Hu, M.L. Hispolon suppresses SK-Hep1 human hepatoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen activator through the PI3K/Akt and ERK signaling pathways. J. Agric. Food Chem. 2010, 58, 9468–9475. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.C.; Zhou, M.; Shankavaram, U.; Peng, G.; Wahl, L.M. Differential Regulation of Lipopolysaccharide-Induced Monocyte Matrix Metalloproteinase (MMP)-1 and MMP-9 by p38 and Extracellular Signal-Regulated Kinase 1/2 Mitogen-Activated Protein Kinases. J. Immunol. 2003, 170, 6244–6249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Gao, W.; Man, S.; Gao, Y.; Huang, L.; Liu, C. Isolation and Identification of Compounds Present in Rhizomes of Paris axialis H. Li and Study of Their Cytotoxic Effects. Lat. Am. J. Pharm. 2011, 30, 540–545. [Google Scholar]
- Zhang, C.; Jia, X.; Bao, J.; Chen, S.; Wang, K.; Zhang, Y.; Li, P.; Wan, J.B.; Su, H.; Wang, Y. Polyphyllin VII induces apoptosis in HepG2 cells through ROS-mediated mitochondrial dysfunction and MAPK pathways. BMC Complement. Altern. Med. 2016, 16, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jia, X.; Wang, K.; Bao, J.; Li, P.; Chen, M.; Wan, J.B.; Su, H.; Mei, Z.; He, C. Polyphyllin VII Induces an Autophagic Cell Death by Activation of the JNK Pathway and Inhibition of PI3K/AKT/mTOR Pathway in HepG2 Cells. PLoS ONE 2016, 11, e0147405. [Google Scholar] [CrossRef] [PubMed]
- Trede, N.S.; Zapata, A.; Zon, L.I. Fishing for lymphoid genes. Trends Immunol. 2001, 22, 302–307. [Google Scholar] [CrossRef]
- Park, K.H.; Cho, K.H. A zebrafish model for the rapid evaluation of pro-oxidative and inflammatory death by lipopolysaccharide, oxidized low-density lipoproteins, and glycated high-density lipoproteins. Fish Shellfish Immunol. 2011, 31, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, S.; Joseph, B.; Kar, B. Anti-inflammatory activity of Withania somnifera leaf extract in stainless steel implant induced inflammation in adult zebrafish. J. Genet. Eng. Biotechnol. 2014, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Quezada, M.; Alvarez, M.; Peña, O.A.; Henríquez, S.; d’Alençon, C.A.; Lange, S.; Oliva, B.; Owen, G.I.; Allende, M.L. Antiangiogenic, antimigratory and antiinflammatory effects of 2-methoxyestradiol in zebrafish larvae. Comp. Biochem. Physiol. Part C 2013, 157, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wang, W.H.; Wang, Y.H.; Lin, Z.Y.; Wen, C.C.; Chern, C.Y. Evaluation of the anti-inflammatory effect of chalcone and chalcone analogues in a zebrafish model. Molecules 2013, 18, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.; Elworthy, S.; Ingham, P.W.; Whyte, M.K. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieschke, G.J.; Oates, A.C.; Crowhurst, M.O.; Ward, A.C.; Layton, J.E. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 2001, 98, 3087–3096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.P.; Harris, C.C. Inflammation and cancer: An ancient link with novel potentials. Int. J. Cancer J. 2010, 121, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Malik, A.B. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L622–L645. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 2003, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 2005, 7, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, N.; Pan, S.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Roburic Acid Suppresses NO and IL-6 Production via Targeting NF-κB and MAPK Pathway in RAW264.7 Cells. Inflammation 2017, 40, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tjäderhane, L.; Konttinen, Y.T.; Lauhio, A.; Salo, T.; Lee, H.M.; Golub, L.M.; Brown, D.L.; Mäntylä, P. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann. Med. 2006, 38, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Lee, Y.C.; Kim, C.H. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: Involvement of invasive potential. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chen, T.; Chen, Z.; Zhang, P.; Pi, H.; Ruan, H.; Wu, J. Anti-inflammatory activity of omphalocarpin isolated from Radix Toddaliae Asiaticae. J. Ethnopharmacol. 2014, 155, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Swingle, K.F.; Shideman, F.E. Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain anti-inflammatory agents. J. Pharmacol. Exp. Ther. 1972, 183, 226–234. [Google Scholar] [PubMed]
- Choi, T.-Y.; Kim, J.-H.; Dong, H.K.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Sun, Y.K.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2010, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, C.; Reischl, M.; Shah, A.H.; Mikut, R.; Liebel, U.; Grabher, C. Facilitating Drug Discovery: An Automated High-content Inflammation Assay in Zebrafish. J. Vis. Exp. Jove 2012, 65, e4203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, D.; Ma, X.; Liu, Z.; Li, F.; Wu, D. Paris saponin VII suppressed the growth of human cervical cancer Hela cells. Eur. J. Med. Res. 2014, 19, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, L.; Sun, Y.; Miao, X.; Zhang, F.; Meng, J.; Han, J.; Zhang, D.; Zhang, R.; Yue, Z. Paris saponin VII from trillium tschonoskii reverses multidrug resistance of adriamycin-resistant MCF-7/ADR cells via P-glycoprotein inhibition and apoptosis augmentation. J. Ethnopharmacol. 2014, 154, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Fan, L.; Zhang, F.; Meng, J.; Han, J.; Guo, X.; Zhang, D.; Zhang, R.; Yue, Z. Paris saponin VII inhibits growth of colorectal cancer cells through Ras signaling pathway. Biochem. Pharm. 2014, 88, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Hotchkiss, J.H. Potential genotoxicity of chronically elevated nitric oxide: A review. Mutat. Res. 1995, 339, 73–89. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Natarajan, K. Tumor necrosis factors: Developments during the last decade. Eur. Cytokine Netw. 1996, 7, 93–124. [Google Scholar] [PubMed]
- Jung, W.K.; Choi, I.; Lee, D.Y.; Yea, S.S.; Choi, Y.H.; Kim, M.M.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-kappaB pathways. Int. J. Biochem. Cell Biol. 2008, 40, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Molloy, R.G.; Mannick, J.A.; Rodrick, M.L. Cytokines, sepsis and immunomodulation. Br. J. Surg. 2010, 80, 289–297. [Google Scholar] [CrossRef]
- Grinbergbleyer, Y.; Ghosh, S. A Novel Link between Inflammation and Cancer. Cancer Cell 2016, 30, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Benneriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.W.; Lawrence, T.; Perretti, M.; Rossi, A.G. Inflammatory resolution: New opportunities for drug discovery. Nat. Rev. Drug Discov. 2004, 3, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Meng, X.L.; Yang, J.Y.; Chen, G.L.; Wang, L.H.; Zhang, L.J.; Wang, S.; Li, J.; Wu, C.F. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem. Biol. Interact. 2008, 174, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.; Larkin, A.; Mingo, A.M.; Thuerauf, D.J.; Andrews, C.; Mcdonough, P.M.; Glembotski, C.C. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 2000, 275, 23814–23824. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, T.; Guo, Z. Curcumin inhibits ox-LDL-induced MCP-1 expression by suppressing the p38MAPK and NF-κB pathways in rat vascular smooth muscle cells. Inflamm. Res. 2012, 61, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.H.; Alkofahi, A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J. Ethnopharmacol. 1998, 60, 117–124. [Google Scholar] [CrossRef]
- Recio, M.C.; Giner, R.M.; Máñez, S.; Ríos, J.L. Structural requirements for the anti-inflammatory activity of natural triterpenoids. Planta Med. 1995, 61, 182–185. [Google Scholar] [CrossRef] [PubMed]
- D’Alençon, C.A.; Peña, O.A.; Wittmann, C.; Gallardo, V.E.; Jones, R.A.; Loosli, F.; Liebel, U.; Grabher, C.; Allende, M.L. A high-throughput chemically induced inflammation assay in zebrafish. BMC Biol. 2010, 8, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.E.; Teixeira, A.D.C.; Cruz, F.F.; Concatto, S.C.; Amaral, J.H.; Bonan, C.D.; Campos, M.M.; Morrone, F.B.; Battastini, A.M.O. Analytical method for determination of nitric oxide in zebrafish larvae: Toxicological and pharmacological applications. Anal. Biochem. 2012, 421, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.X.; Zhang, W.Y.; Shen, W.B.; Wang, K.C. Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cell. Immunol. 2010, 262, 69–74. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, K.; Wang, S.; Hou, H.; Yuan, Y.; Wang, X. Toxicity induced by emodin on zebrafish embryos. Drug Chem. Toxicol. 2012, 35, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, C.; Song, L.; Li, X.; Shi, S.; Mo, J.; Chen, H.; Bai, H.; Wu, X.; Zhao, J. Effect of total phenolics from Laggera alata on acute and chronic inflammation models. J. Ethnopharmacol. 2006, 108, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.R.; Perimal, E.K.; Akhtar, M.N.; Mohamad, A.; Khalid, M.H.; Tasrip, N.A.; Mokhtar, F.; Zakaria, Z.A.; Lajis, N.; Israf, D. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia 2010, 81, 855–858. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of PP7 are not available from the authors. |






| Group | Dose (mg/kg) | Edema Degree (mg) | Inhibition Rate (%) |
|---|---|---|---|
| Control | - | 6.26 ± 0.59 | - |
| DEX | 5 | 2.65 ± 0.86 ** | 57.6 |
| PP7 | 0.5 | 5.60 ± 0.25 * | 10.6 |
| PP7 | 1 | 4.28 ± 0.32 ** | 31.6 |
| PP7 | 2 | 3.78 ± 0.31 ** | 39.7 |
| Group | Dose (mg/kg) | Granuloma Dry Weight (mg) | Inhibition Rate (%) |
|---|---|---|---|
| Control | - | 28.69 ± 3.23 | - |
| DEX | 5 | 12.17 ± 2.30 ** | 57.59 |
| PP7 | 0.5 | 19.33 ± 2.80 ** | 32.61 |
| PP7 | 1 | 13.83 ± 2.52 ** | 51.78 |
| PP7 | 2 | 12.88 ± 2.31 ** | 55.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, C.; Jia, X.; Wang, K.; Tu, Y.; Wang, R.; Liu, K.; Lu, T.; He, C. In Vitro and In Vivo Anti-Inflammatory Effects of Polyphyllin VII through Downregulating MAPK and NF-κB Pathways. Molecules 2019, 24, 875. https://doi.org/10.3390/molecules24050875
Zhang C, Li C, Jia X, Wang K, Tu Y, Wang R, Liu K, Lu T, He C. In Vitro and In Vivo Anti-Inflammatory Effects of Polyphyllin VII through Downregulating MAPK and NF-κB Pathways. Molecules. 2019; 24(5):875. https://doi.org/10.3390/molecules24050875
Chicago/Turabian StyleZhang, Chao, Chaoying Li, Xuejing Jia, Kai Wang, Yanbei Tu, Rongchun Wang, Kechun Liu, Tao Lu, and Chengwei He. 2019. "In Vitro and In Vivo Anti-Inflammatory Effects of Polyphyllin VII through Downregulating MAPK and NF-κB Pathways" Molecules 24, no. 5: 875. https://doi.org/10.3390/molecules24050875