Preparation of Poly (Allylthiourea-Co-Acrylic Acid) Derived Carbon Materials and Their Applications in Wastewater Treatment
Abstract
:1. Introduction
2. Results
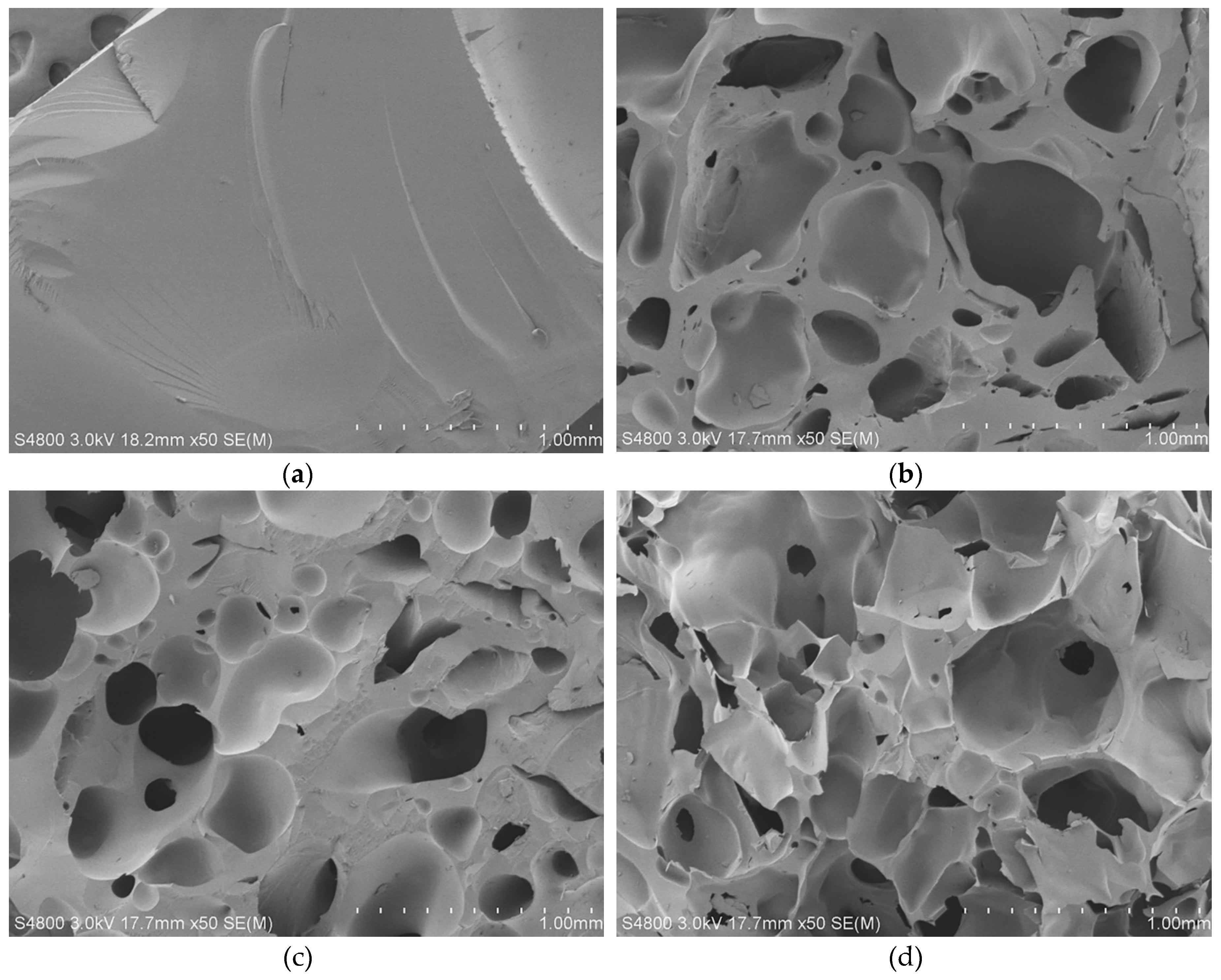
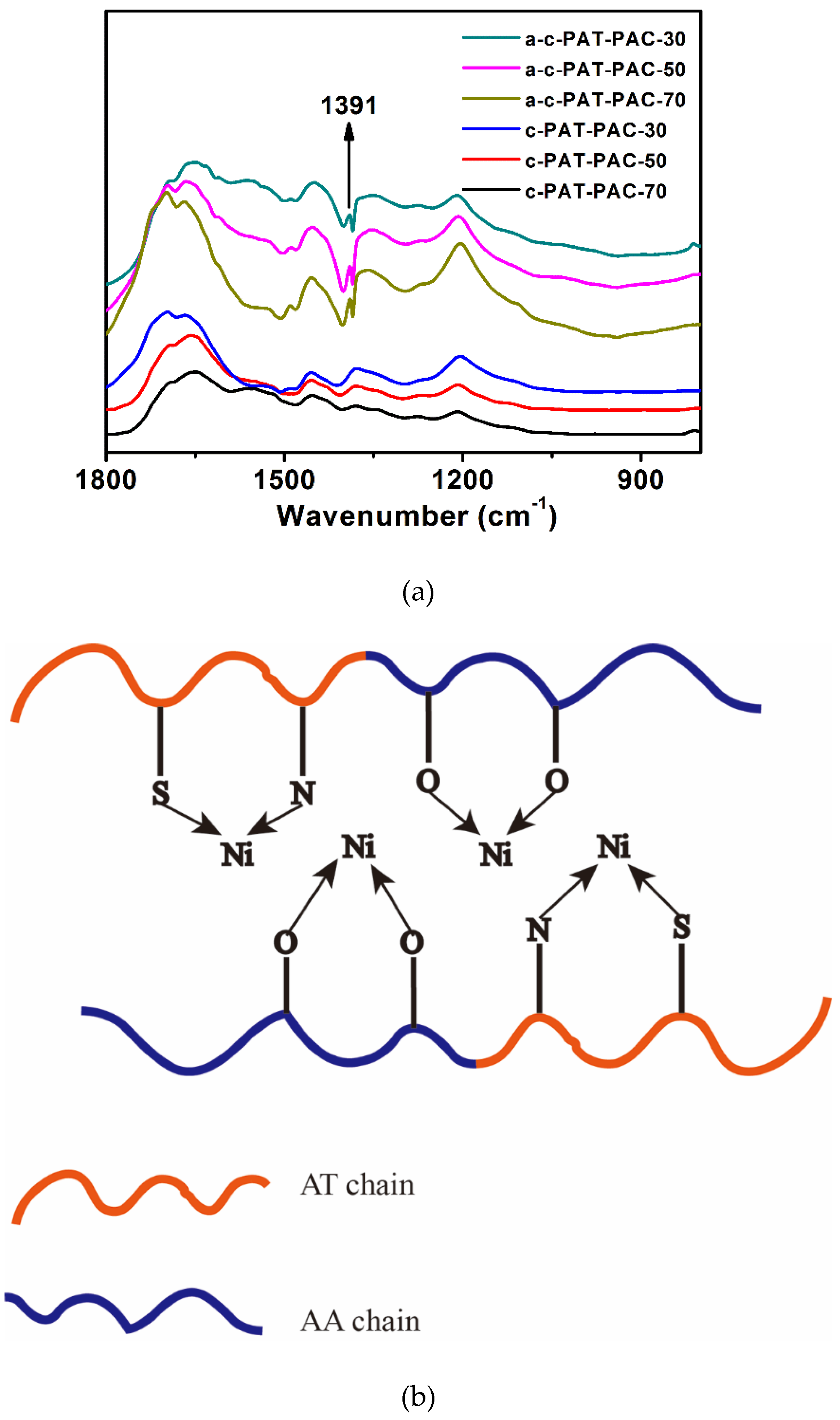
2.1. Characterizations
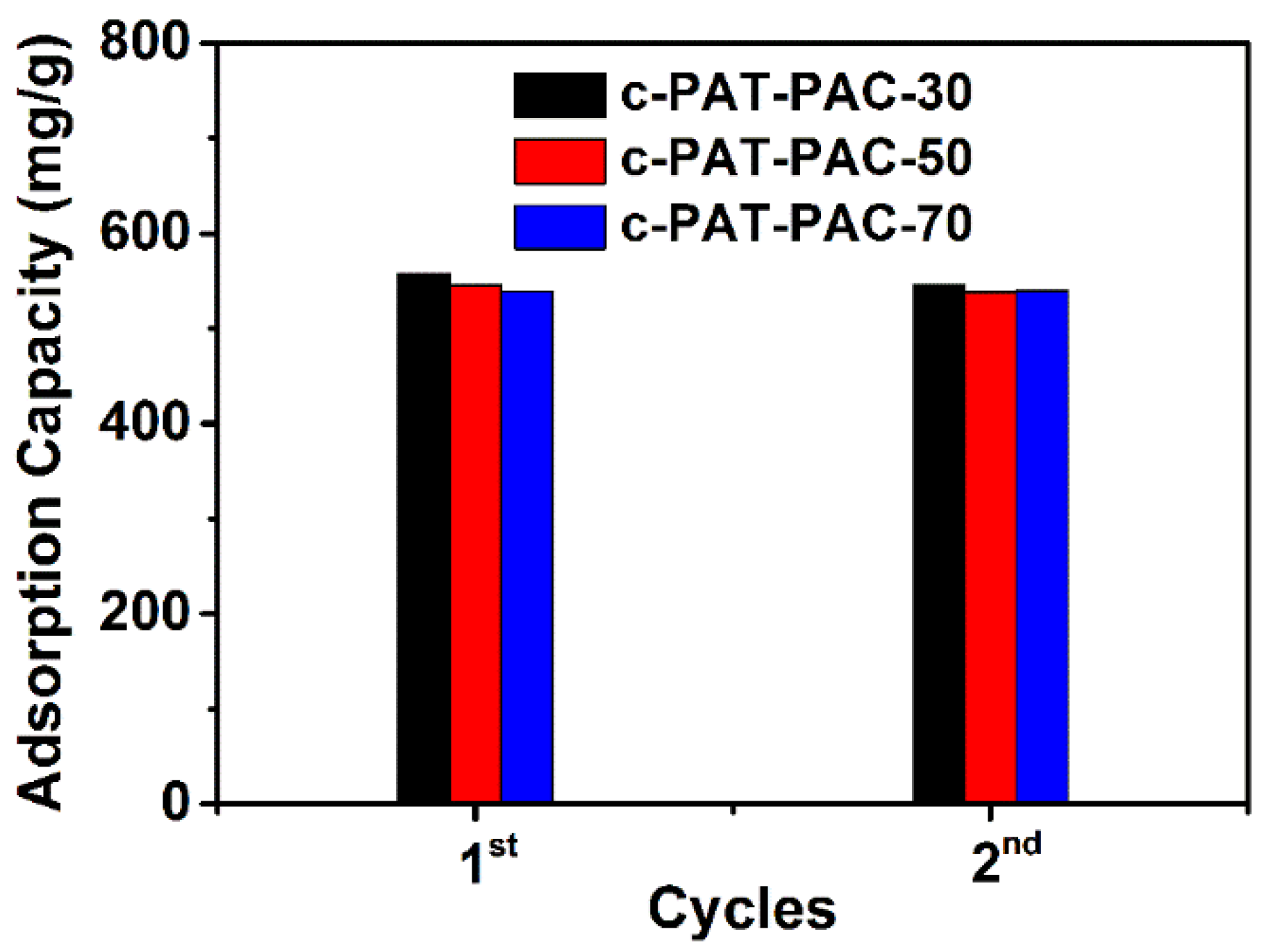
2.2. Heavy Metal Ion Adsorption
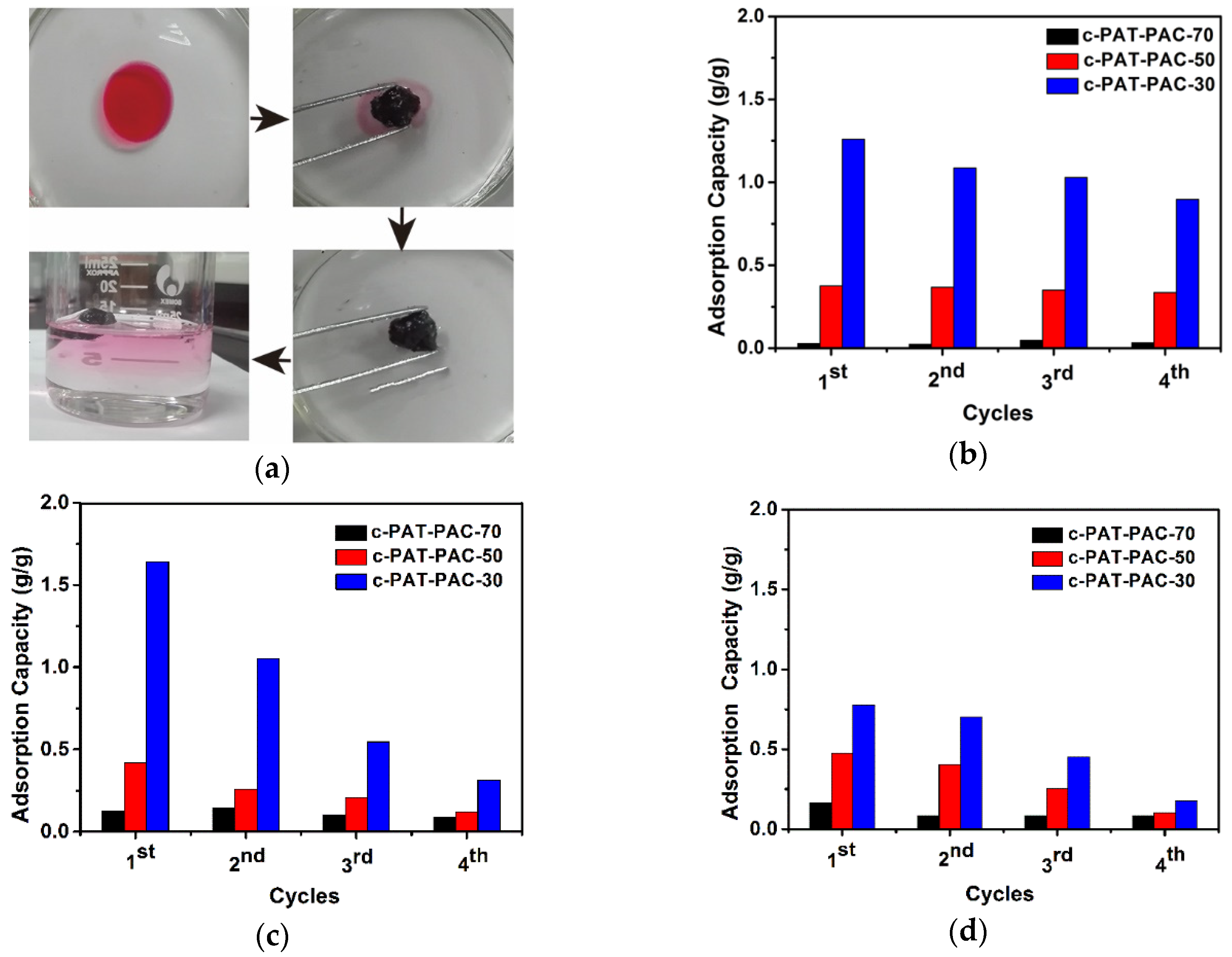
2.3. Organic Solvent and Dye Adsorption
3. Materials and Methods
3.1. Reagents
3.2. Hydrogel Preparations
3.3. Characterizations
3.4. Heavy Metal Ion Adsorption
3.5. Organic Solvent and Dye Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miroshnychenko, I.; Barontini, R.; Testa, F. Green practices and financial performance: A global outlook. J. Clean. Prod. 2017, 147, 340–351. [Google Scholar] [CrossRef]
- Visa, M.; Bogatu, C.; Duta, A. Simultaneous adsorption of dyes and heavy metals from multicomponent solutions using fly ash. Appl. Surf. Sci. 2010, 256, 5486–5491. [Google Scholar] [CrossRef]
- Cheng, Z.F.; Wu, Y.H.; Wang, N.; Yang, W.H.; Xu, T.W. Development of a Novel Hollow Fiber Cation-Exchange Membrane from Bromomethylated Poly(2,6-dimethyl-1,4-phenylene oxide) for Removal of Heavy-Metal Ions. Ind. Eng. Chem. Res. 2010, 49, 3079–3087. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B.X. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.H.; Wang, D.; Liu, X.; Pei, X.F.; Chen, L.W.; Jin, J. Bio-inspired surface-functionalization of graphene oxide for the adsorption of organic dyes and heavy metal ions with a superhigh capacity. J. Mater. Chem. A 2014, 2, 5034–5040. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, Y.; Wang, A. Kapok fiber structure-oriented polyallylthiourea: Efficient adsorptive reduction for Au(III) for catalytic application. Polymer 2014, 55, 5211–5217. [Google Scholar] [CrossRef]
- Qu, W.S.; Zhai, Y.H.; Meng, S.M.; Fan, Y.Q.; Zhao, Q. Selective solid phase extraction and preconcentration of trace mercury(II) with poly-allylthiourea packed columns. Microchim. Acta 2008, 163, 277–282. [Google Scholar] [CrossRef]
- Inam, R.; Caykara, T.; Ozcan, S.; Yagmur, M. Polarographic determination of Pb(II) and Cd(II) with selective removal of Se(IV) using ionic poly(N,N-dimethylacrylamide-co-allylthiourea). J. Anal. Chem. 2005, 60, 1139–1143. [Google Scholar] [CrossRef]
- Kilic, A.G.; Malci, S.; Celikbicak, O.; Sahiner, N.; Salih, B. Gold recovery onto poly(acrylamide-allylthiourea) hydrogels synthesized by treating with gamma radiation. Anal. Chim. Acta 2005, 547, 18–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Luo, X. Facile preparation of a novel Hg(II)-ion-imprinted polymer based on magnetic hybrids for rapid and highly selective removal of Hg(II) from aqueous solutions. RSC Adv. 2016, 6, 14916–14926. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A. Granular hydrogel initiated by Fenton reagent and their performance on Cu(II) and Ni(II) removal. Chem. Eng. J. 2012, 200, 601–610. [Google Scholar] [CrossRef]
- Liu, Q.; Ke, M.; Liu, F.; Yu, P.; Hu, H.; Li, C. High-performance removal of methyl mercaptan by nitrogen-rich coconut shell activated carbon. RSC Adv. 2017, 7, 22892–22899. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, X.X.; Li, J.X.; Wang, X.K. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J. Mater. Chem. A 2015, 3, 6073–6081. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gong, Y.T.; Wang, Y. Cellulose-based hydrophobic carbon aerogels as versatile and superior adsorbents for sewage treatment. RSC Adv. 2014, 4, 45753–45759. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, S.C.; Liu, G.Z.; Yao, J.F. Facile and fast removal of oil through porous carbon spheres derived from the fruit of Liquidambar formosana. Chemosphere 2017, 170, 68–74. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, P.; Wang, M.; Wu, G.; Dong, C.; Wu, A. Biomass-Derived Porous Carbonaceous Aerogel as Sorbent for Oil-Spill Remediation. ACS Appl. Mater. Interfaces 2016, 8, 32862–32868. [Google Scholar] [CrossRef]
- Basar, C.A. Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J. Hazard. Mater. 2006, 135, 232–241. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Zhang, L.; Tian, Y.; Cui, G.; Yan, S. A cost-effective porous carbon derived from pomelo peel for the removal of methyl orange from aqueous solution. Colloids Surf. A 2016, 489, 191–199. [Google Scholar] [CrossRef]
- Wang, J.C.; Senkovska, I.; Oschatz, M.; Lohe, M.R.; Borchardt, L.; Heerwig, A.; Liu, Q.; Kaskel, S. Imine-Linked Polymer-Derived Nitrogen-Doped Microporous Carbons with Excellent CO2 Capture Properties. ACS Appl. Mater. Interfaces 2013, 5, 3160–3167. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid Synthesis of Nitrogen-Doped Porous Carbon Monolith for CO2 Capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Q.; Zhang, S.Z.; He, W.; Zhang, X.D.; Yang, G.H.; Zhang, J.; Shi, X.Y.; Wang, L.Z. SiO2-carbon nanocomposite anodes with a 3D interconnected network and porous structure from bamboo leaves. RSC Adv. 2016, 6, 1930–1937. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, B.; Ma, M.; Wu, B.; Shi, Y.; Wang, X. Multiporous microstructure for enhancing the water absorption and swelling rate in poly(sodium acrylic acid) superabsorbent hydrogels based on a novel physical and chemical composite foaming system. J. Appl. Polym. Sci. 2016, 133, 44149–44155. [Google Scholar] [CrossRef]
- Song, P.A.; Xu, Z.; Dargusch, M.S.; Chen, Z.-G.; Wang, H.; Guo, Q. Granular Nanostructure: A Facile Biomimetic Strategy for the Design of Supertough Polymeric Materials with High Ductility and Strength. Adv. Mater. 2017, 29, 1704661–1704668. [Google Scholar] [CrossRef]
- Somani, P.R.; Marimuthu, R.; Viswanath, A.K.; Radhakrishnan, S. Thermal degradation properties of solid polymer electrolyte (poly(vinyl alcohol) plus phosphoric acid)/methylene blue composites. Polym. Degrad. Stab. 2003, 79, 77–83. [Google Scholar] [CrossRef]
- Qian, M.; Sun, Y.; Xu, X.; Liu, L.; Song, P.; Yu, Y.; Wang, H.; Qian, J. 2D-alumina platelets enhance mechanical and abrasion properties of PA612 via interfacial hydrogen-bond interactions. Chem. Eng. J. 2017, 308, 760–771. [Google Scholar] [CrossRef]
- Li, C.P.; Hou, T.T.; She, X.D.; Wei, X.Y.; She, F.H.; Gao, W.M.; Kong, L.X. Decomposition properties of PVA/graphene composites during melting-crystallization. Polym. Degrad. Stab. 2015, 119, 178–189. [Google Scholar] [CrossRef]
- Li, C.P.; Vongsvivut, J.; She, X.D.; Li, Y.Z.; She, F.H.; Kong, L.X. New insight into non-isothermal crystallization of PVA-graphene composites. Phys. Chem. Chem. Phys. 2014, 16, 22145–22158. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Zhang, J. Preparation of high adsorption performance activated carbon by pyrolysis of waste polyester fabric. J. Mater. Sci. 2018, 53, 5458–5466. [Google Scholar] [CrossRef]
- Li, C.P.; Zhu, H.J.; Salim, N.V.; Fox, B.L.; Hameed, N. Preparation of microporous carbon materials via in-depth sulfonation and stabilization of polyethylene. Polym. Degrad. Stab. 2016, 134, 272–283. [Google Scholar] [CrossRef]
- Zhu, Q.F.; Zhang, B.B.; Wang, T.T.; Feng, X.D. Synthesis and properties of porous delta-MnO2/polymer millimeter-sized beads for Ni(II) removal. Microporous Mesoporous Mater. 2019, 273, 90–98. [Google Scholar] [CrossRef]
- Sargin, I.; Arslan, G. Chitosan/sporopollenin microcapsules: Preparation, characterisation and application in heavy metal removal. Int. J. Biol. Macromol. 2015, 75, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Wei, Y.Z.; Mira, H.I.; Abdel-Rahman, A.A.H.; Guibal, E. Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni(II) and Pb(II) recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef]
- Dolgormaa, A.; Lv, C.J.; Li, Y.; Yang, J.; Yang, J.X.; Chen, P.; Wang, H.P.; Huang, J. Adsorption of Cu(II) and Zn(II) Ions from Aqueous Solution by Gel/PVA-Modified Super-Paramagnetic Iron Oxide Nanoparticles. Molecules 2018, 23, 2982. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, Y.L.; Lu, W.P.; Guo, Y.C.; Zhu, Y.; Lu, H.J.; Song, Y.P. Environmentally Friendly Gelatin/beta-Cyclodextrin Composite Fiber Adsorbents for the Efficient Removal of Dyes from Wastewater. Molecules 2018, 23, 2473. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef] [Green Version]
- Kilic, M.; Apaydin-Varol, E.; Putun, A.E. Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2011, 189, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kong, X.; Zhang, C.; Chen, Y.; Hua, Y. Adsorption of soy isoflavones by activated carbon: Kinetics, thermodynamics and influence of soy oligosaccharides. Chem. Eng. J. 2013, 215, 113–121. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, S.; Liu, L.; Shao, Q.; Mai, X.; Zhao, C.X.; Hao, L.; Wu, T.; Yu, Z.; Guo, Z. Microwave Solvothermal Fabrication of Zirconia Hollow Microspheres with Different Morphologies Using Pollen Templates and Their Dye Adsorption Removal. Ind. Eng. Chem. Res. 2018, 57, 231–241. [Google Scholar] [CrossRef]
- Wang, F.; Pan, Y.; Cai, P.; Guo, T.; Xiao, H. Single and binary adsorption of heavy metal ions from aqueous solutions using sugarcane cellulose-based adsorbent. Bioresour. Technol. 2017, 241, 482–490. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Gajbe, V.; Mittal, J. Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: Bottom ash and de-oiled soya. Ind. Eng. Chem. Res. 2006, 45, 1446–1453. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Yu, B.; Tan, Q.; Zhang, X.; Cong, H. Advanced Modified Polyacrylonitrile Membrane with Enhanced Adsorption Property for Heavy Metal Ions. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Verma, N. Surface ion imprinting-mediated carbon nanofiber-grafted highly porous polymeric beads: Synthesis and application towards selective removal of aqueous Pb(II). Chem. Eng. J. 2017, 313, 1142–1151. [Google Scholar] [CrossRef]
- Han, S.; Sun, Q.; Zheng, H.; Li, J.; Jin, C. Green and facile fabrication of carbon aerogels from cellulose-based waste newspaper for solving organic pollution. Carbohydr. Polym. 2016, 136, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Han, S.; Li, J.; Sun, Q. Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohydr. Polym. 2015, 123, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Rashidzadeh, A.; Olad, A.; Salari, D. The Effective Removal of Methylene Blue Dye from Aqueous Solutions by NaAlg-g-Poly(acrylic acid-co-acryl amide)/Clinoptilolite Hydrogel Nanocomposite. Fibers Polym. 2015, 16, 354–362. [Google Scholar] [CrossRef]
- Bekiari, V.; Sotiropoulou, M.; Bokias, G.; Lianos, P. Use of poly(N,N-dimethylacrylamide-co-sodium acrylate) hydrogel to extract cationic dyes and metals from water. Colloids Surf. A 2008, 312, 214–218. [Google Scholar] [CrossRef]
- Li, C.P.; She, M.; She, X.D.; Dai, J.E.; Kong, L.X. Functionalization of Polyvinyl Alcohol Hydrogels with Graphene Oxide for Potential Dye Removal. J. Appl. Polym. Sci. 2014, 131, 39872–39880. [Google Scholar] [CrossRef]
- Hou, P.C.; Xing, G.J.; Tian, L.Y.; Zhang, G.; Wang, H.; Yu, C.N.; Li, Y.L.; Wu, Z.L. Hollow carbon spheres/graphene hybrid aerogels as high-performance adsorbents for organic pollution. Sep. Purif. Technol. 2019, 213, 524–532. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Xu, G.; Pei, Y. Effective removing of methylene blue from aqueous solution by tannins immobilized on cellulose microfibers. Int. J. Biol. Macromol. 2019, 129, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.; Selim, A.Q.; Zayed, A.M.; Komarneni, S.; Mobarak, M.; Seliem, M.K. Enhancing adsorption capacity of Egyptian diatomaceous earth by thermo-chemical purification: Methylene blue uptake. J. Colloid Interface Sci. 2019, 534, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Kasmi, T.; Soualah, A.; Mignard, S.; Batonneau-Gener, I. Effect of BrOnsted acidity of HY zeolites in adsorption of methylene blue and comparative study with bentonite. J. Environ. Health Sci. Eng. 2018, 16, 239–247. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |






| Samples | C (at.%) | O (at.%) | S (at.%) | N (at.%) |
|---|---|---|---|---|
| PAT–PAC-70 | 65.98 | 24.04 | 2.98 | 7.0 |
| PAT–PAC-30 | 66.66 | 13.46 | 6.16 | 13.72 |
| c-PAT–PAC-70 | 74.91 | 13.78 | 1.23 | 10.07 |
| c-PAT–PAC-30 | 75.02 | 8.26 | 2.10 | 14.63 |
| Adsorbents | Adsorption Capability (mg/g) | Reference |
|---|---|---|
| c-PAT–PAC-30 | 557 (pH = 4.0, 30 °C) | This work |
| δ-MnO2/polymer millimeter-sized bead | 29 (pH = 4.0, 30 °C) | [31] |
| chitosan/sporopollenin microcapsules | 13 (pH = 4.0, 25 °C) | [32] |
| grafted hydrazinyl amine magnetite–chitosan | 82 (pH = 4.0, 25 °C) | [33] |
| Adsorbent | Pseudo First-Order Model | Pseudo Second-Order Model | |||
|---|---|---|---|---|---|
| K1 (min−1) | R2 | K2 (g mg−1 min−1) | h (mg/g min) | R2 | |
| c-PAT–PAC-30 | 0.0388 | 0.6002 | 0.0004 | 416.7 | 0.9983 |
| c-PAT–PAC-50 | 0.0626 | 0.8566 | 0.0003 | 416.7 | 0.9990 |
| c-PAT–PAC-70 | 0.0405 | 0.7062 | 0.0005 | 666.7 | 0.9995 |
| Adsorbent | Langmuir Model | Freundlich Model | Tempkin Model | |||||
|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | b (L mg−1) | R2 | n | Kf (L g−1) | R2 | A (L mg−1) | R2 | |
| c-PAT–PAC-30 | –2500 | –6141 | 0.3227 | 0.9655 | 2.2648 | 0.9940 | 0.0636 | 0.9085 |
| c-PAT–PAC-50 | 2500 | 6397 | 0.0611 | 1.0240 | 2.6735 | 0.9657 | 0.0731 | 0.9508 |
| c-PAT–PAC-70 | –25,000 | –82,183 | 0.0027 | 0.9748 | 2.9988 | 0.9875 | 0.0692 | 0.9613 |
| Adsorbents | Adsorption Capability (mg/g) | Reference |
|---|---|---|
| PAT–PAC-30 | 39 (pH = 5.27, 30 °C) | Present study |
| c-PAT–PAC-30 | 110 (pH = 5.27, 30 °C) | Present study |
| tannin-supported on cellulose microfibers | 12 (pH = 5.25, 30 °C) | [51] |
| purified diatomite via thermo–chemical treatment | 28.5 (pH = 5.25, 25 ± 2 °C) | [52] |
| HY zeolites (HY (16.6)) | 100 (pH = 5.25, 25 °C) | [53] |
| Hydrogel | AA/ mol | AT/ mol | IPA/ g | EGDMA/ mol | AIBN/ mol |
|---|---|---|---|---|---|
| PAT–PAC-70 | 0.07 | 0.03 | 18 | 0.01 | 0.005 |
| PAT–PAC-50 | 0.05 | 0.05 | 18 | 0.01 | 0.005 |
| PAT–PAC-30 | 0.03 | 0.07 | 18 | 0.01 | 0.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Li, C.; Hou, T.; Zhong, Z.; Chen, D.; Li, S.; Hu, Z.; Yang, H.; Ye, X. Preparation of Poly (Allylthiourea-Co-Acrylic Acid) Derived Carbon Materials and Their Applications in Wastewater Treatment. Molecules 2019, 24, 957. https://doi.org/10.3390/molecules24050957
Liang L, Li C, Hou T, Zhong Z, Chen D, Li S, Hu Z, Yang H, Ye X. Preparation of Poly (Allylthiourea-Co-Acrylic Acid) Derived Carbon Materials and Their Applications in Wastewater Treatment. Molecules. 2019; 24(5):957. https://doi.org/10.3390/molecules24050957
Chicago/Turabian StyleLiang, Limei, Chengpeng Li, Tingting Hou, Zhiying Zhong, Dongchu Chen, Sidong Li, Zhang Hu, Haihua Yang, and Xiufang Ye. 2019. "Preparation of Poly (Allylthiourea-Co-Acrylic Acid) Derived Carbon Materials and Their Applications in Wastewater Treatment" Molecules 24, no. 5: 957. https://doi.org/10.3390/molecules24050957





