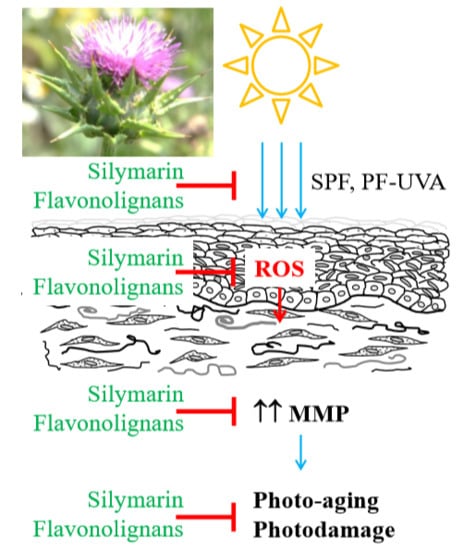
Skin Protective Activity of Silymarin and its Flavonolignans
Abstract
:1. Introduction
2. Results
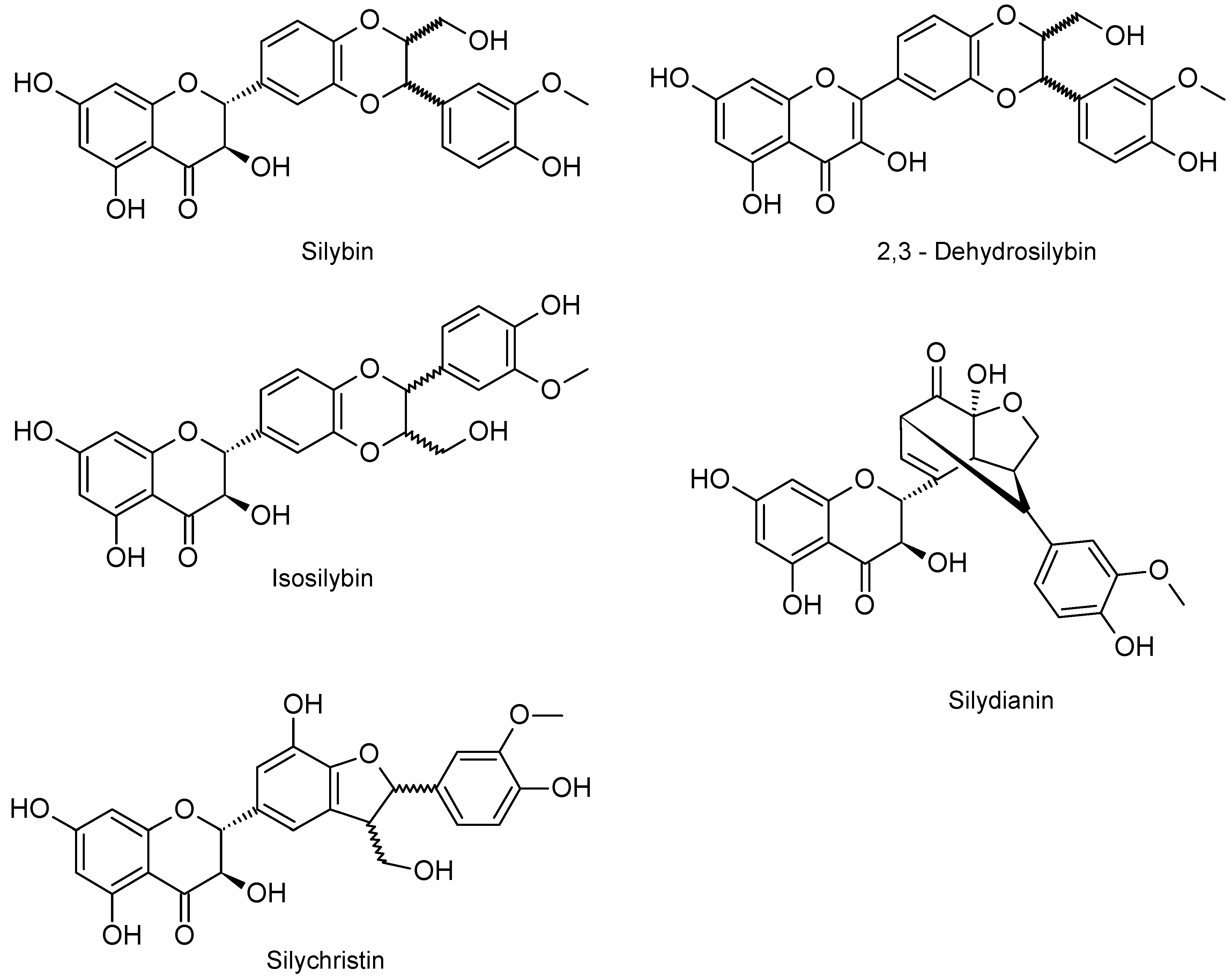
2.1. Chemical Composition of Silymarin
2.2. DPPH Scavenging Activity
2.3. UV Absorption Ability
2.4. Sun and UVA Protection Factors
2.5. Effect on Enzymes Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Silymarin and its Flavonolignans
4.3. DPPH Scavenging Activity
4.4. Measurement of Spectra
4.5. Sun and UVA Protection Factors
4.6. Anti-Aging Potential of Silymarin and Flavonolignans
4.6.1. Anti-Elastase Activity
4.6.2. Anti-Collagenase Activity
4.6.3. Anti-Hyaluronidase Activity
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Rijken, F.; Bruijnzeel, P.L. The pathogenesis of photoaging: The role of neutrophils and neutrophil-derived enzymes. J. Investig. Dermatol. Symp. Proc. 2009, 14, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.P.; Bölke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2016, 21, 5. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential role of natural compounds against skin aging. Curr Med Chem 2015, 22, 1515–1538. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition...and why does it matter? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Katiyar, S.K. Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn.) (Review). Int. J. Oncol. 2010, 36, 1053–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [Green Version]
- Gažák, R.; Svobodová, A.; Psotová, J.; Sedmera, P.; Přikrylová, V.; Walterová, D.; Kren, V. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorg. Med. Chem. 2004, 12, 5677–5687. [Google Scholar] [CrossRef]
- Rajnochová Svobodová, A.; Gabrielová, E.; Michaelides, L.; Kosina, P.; Ryšavá, A.; Ulrichová, J.; Zálešák, B.; Vostálová, J. UVA-photoprotective potential of silymarin and silybin. Arch. Dermatol. Res. 2018, 310, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Katiar, S.K. Silymarin and skin cancer prevention: Anti-inflammatory, antioxidant and immunomodulatory effects (Review). Int. J. Oncol. 2005, 26, 169–176. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.P.; Saleh, M.A. Free radical scavenging and antioxidant activities of silymarin components. Antioxidants 2013, 2, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.; Gülçin, I.; Beyza, S.; Sarikaya, O.; Bursal, E. In vitro antioxidant activity of silymarin. J. Enzyme Inhib. Med. Chem. 2009, 24, 395–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Zdařilová, A.; Malisková, J.; Mikulková, H.; Walterová, D.; Vostalová, J. Attenuation of UVA-induced damage to human keratinocytes by silymarin. J. Dermatol. Sci. 2007, 46, 21–30. [Google Scholar] [CrossRef]
- Svobodová, A.; Zdařilová, A.; Walterová, D.; Vostálová, J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J. Dermatol. Sci. 2007, 48, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Vimalraj, S.; Rajalakshmi, S.; Saravanan, S.; Raj Preeth, D.; LA Vasanthi, R.; Shairam, M.; Chatterjee, S. Synthesis and characterization of zinc-silibinin complexes: A potential bioactive compound with angiogenic, and antibacterial activity for bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 167, 134–143. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Svobodová, A.; Vostálová, J. Solar radiation induced skin damage: Review of protective and preventive options. Int. J. Radiat. Biol. 2010, 86, 999–1030. [Google Scholar] [CrossRef]
- Couteau, C.; Cheignon, C.; Paparis, E.; Coiffard, L.J. Silymarin, a molecule of interest for topical photoprotection. Nat. Prod. Res. 2012, 26, 2211–2214. [Google Scholar] [CrossRef] [PubMed]
- Rajnochová Svobodová, A.; Zálešák, B.; Biedermann, D.; Ulrichová, J.; Vostálová, J. Phototoxic potential of silymarin and its bioactive components. J. Photochem. Photobiol. B 2016, 156, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Křenek, K.; Marhol, P.; Peikerová, Ž.; Křen, V.; Biedermann, D. Preparatory separation of the silymarin flavonolignans by Sephadex LH-20 gel. Food Res. Int. 2014, 65, 115–120. [Google Scholar] [CrossRef]
- Gažák, R.; Trouillas, P.; Biedermann, D.; Fuksová, K.; Marhol, P.; Kuzma, M.; Kren, V. Base-catalyzed oxidation of silybin and isosilybin into 2,3-dehydro derivatives. Tetrahedron Lett. 2013, 54, 315–317. [Google Scholar] [CrossRef]
- Kosina, P.; Paloncýová, M.; Rajnochová Svobodová, A.; Zálešák, B.; Biedermann, D.; Ulrichová, J.; Vostálová, J. Dermal Delivery of Selected Polyphenols from Silybum marianum. Theoretical and Experimental Study. Molecule 2019, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Agin, P.P.; Levee, G.I.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Diffey, B.L.; Robson, J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Couteau, C.; Couteau, O.; Alami-El Boury, S.; Coiffard, L.J. Sunscreen products: What do they protect us from? Int. J. Pharm. 2011, 415, 181–184. [Google Scholar] [CrossRef]
- Karsili, T.N.; Marchetti, B.; Ashfold, M.N.; Domcke, W. Ab initio study of potential ultrafast internal conversion routes in oxybenzone, caffeic acid, and ferulic acid: Implications for sunscreens. J. Phys. Chem. A 2014, 118, 11999–12010. [Google Scholar] [CrossRef]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complement Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef]
- Maity, N.; Nema, N.K.; Sarkar, B.K.; Mukherjee, P.K. Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor. Indian J. Pharmacol. 2012, 44, 584–587. [Google Scholar] [CrossRef]
Sample Availability: Studied compounds are available from the authors. |



| Compound | Retention Time (min) | Content (%) |
|---|---|---|
| SB A | 6.41 | 16.34 ± 1.60 |
| SB B | 6.99 | 21.64 ± 1.53 |
| ISB A | 8.15 | 5.73 ± 1.16 |
| ISB B | 8.44 | 2.90 ± 0.65 |
| SC A | 3.10 | 13.73 ± 1.20 |
| SC B | 3.82 | 1.83 ± 0.15 |
| SD | 3.68 | 4.55 ± 0.62 |
| DHSB | 12.47 | 0.33 ± 0.07 |
| DHSC | 8.02 | 0.56 ± 0.09 |
| TA | 1.99 | 2.09 ± 0.41 |
| Compound | IC50 (µM) | IC50 (mg/L) |
|---|---|---|
| SB | 527.86 ± 9.75 #,† | 254.43 ± 4.70 *,† |
| DHSB | 26.25 ± 1.75 † | 12.60 ± 0.84 *,† |
| ISB | 251.93 ± 6.56 #,† | 121.43 ± 3.16 *,† |
| SD | 52.12 ± 1.93 #,† | 25.12 ± 0.93 † |
| SC | 38.05 ± 2.14 #,† | 18.34 ± 1.03 *,† |
| SM | - | 25.38 ± 0.97 † |
| QE | 6.75 ± 0.87 | 2.04 ± 0.26 |
| Compounds | SPF(290–320) | SPF(290–400) | UVA-PF |
|---|---|---|---|
| SM a | 5.50 ± 0.25 # | 2.49 ± 0.15 # | 1.52 ± 0.10 # |
| SB a | 6.07 ± 0.19 # | 2.62 ± 0.16 # | 1.50 ± 0.11 # |
| DHSB | 3.64 ± 0.19 # | 2.38 ± 0.12 # | 2.90 ± 0.18 |
| ISB | 5.99 ± 0.11 # | 2.52 ± 0.14 # | 1.45 ± 0.13 # |
| SD | 4.35 ± 0.35 # | 2.01 ± 0.19 # | 1.31 ± 0.13 # |
| SC | 5.66 ± 0.14 # | 2.46 ± 0.16 # | 1.47 ± 0.14 # |
| FA | 7.51 ± 0.16 | 5.13 ± 0.28 | 3.36 ± 0.29 |
| Compounds | Elastase | Collagenase | ||
|---|---|---|---|---|
| IC50 (µM) | IC50 (mg/L) | IC50 (µM) | IC50 (mg/L) | |
| SB | 122.6 ± 4.8 # | 59.1 ± 2.3 * | 52.2 ± 5.0 # | 25.2 ± 2.4 * |
| DHSB | 8.6 ± 0.5 # | 4.1 ± 0.2 * | 23.4 ± 2.9 # | 11.2 ± 1.4 * |
| ISB | ~ | ~ | 50.8 ± 4.5 # | 24.5 ± 2.2 * |
| SD | ~ | ~ | 190.3 ± 4.4 # | 91.8 ± 2.1 * |
| SC | ~ | ~ | 95.9 ± 5.7 # | 46.3 ± 2.8 * |
| SM | - | 6.27 ± 0.4 | - | 2.0 ± 0.3 |
| OA | 10.8 ± 0.6 | 4.9 ± 0.3 * | N.D. | N.D. |
| 1,10-Ph | N.D. | N.D. | 161.3 ± 4.6 | 29.1 ± 0.8 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Rajnochová Svobodová, A. Skin Protective Activity of Silymarin and its Flavonolignans. Molecules 2019, 24, 1022. https://doi.org/10.3390/molecules24061022
Vostálová J, Tinková E, Biedermann D, Kosina P, Ulrichová J, Rajnochová Svobodová A. Skin Protective Activity of Silymarin and its Flavonolignans. Molecules. 2019; 24(6):1022. https://doi.org/10.3390/molecules24061022
Chicago/Turabian StyleVostálová, Jitka, Eva Tinková, David Biedermann, Pavel Kosina, Jitka Ulrichová, and Alena Rajnochová Svobodová. 2019. "Skin Protective Activity of Silymarin and its Flavonolignans" Molecules 24, no. 6: 1022. https://doi.org/10.3390/molecules24061022