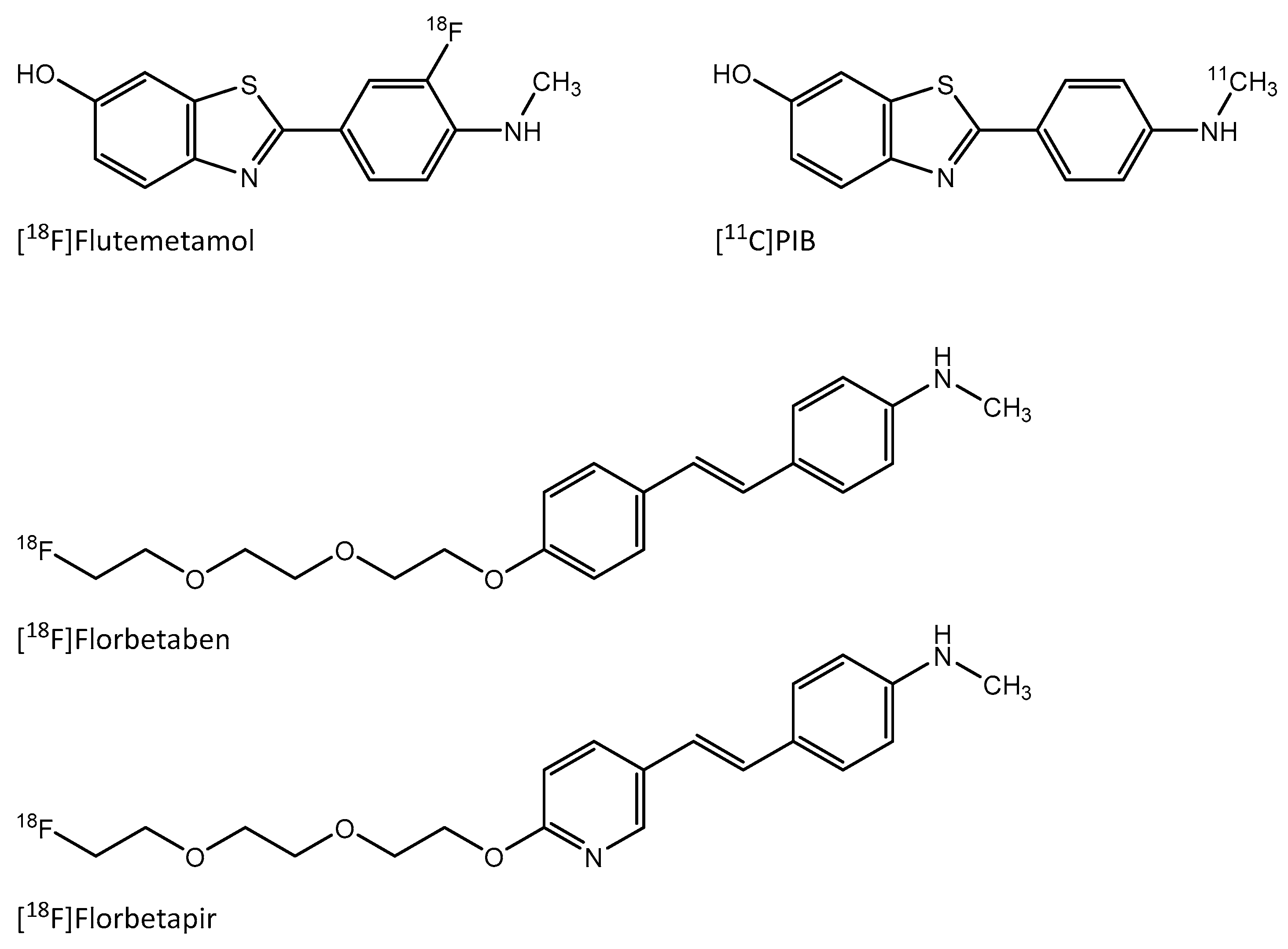
Amyloid-Targeting PET Tracer [18F]Flutemetamol Accumulates in Atherosclerotic Plaques
Abstract
:1. Introduction
2. Results
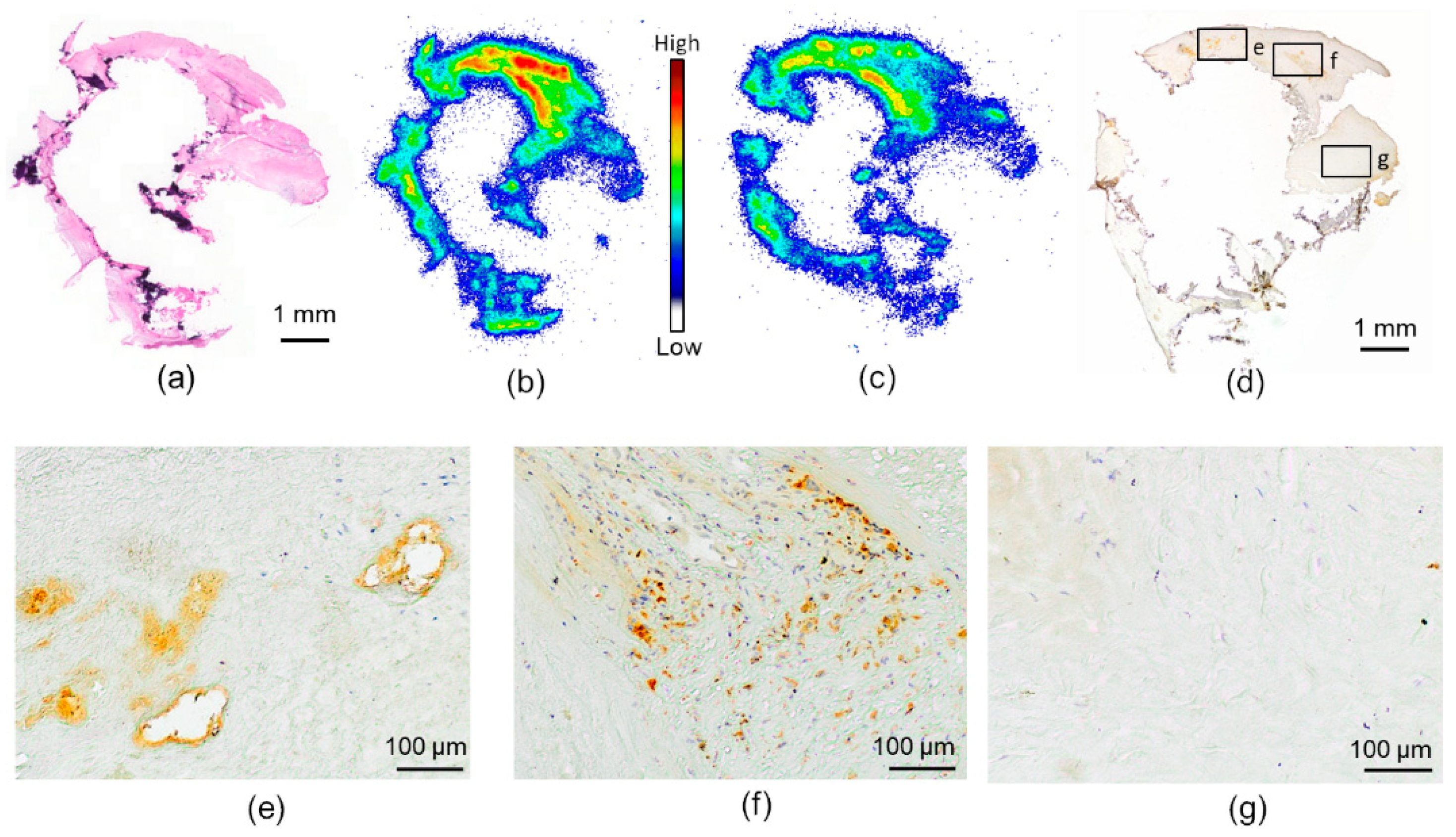
2.1. In Vitro Binding of [18F]Flutemetamol in Human Atherosclerotic Plaque
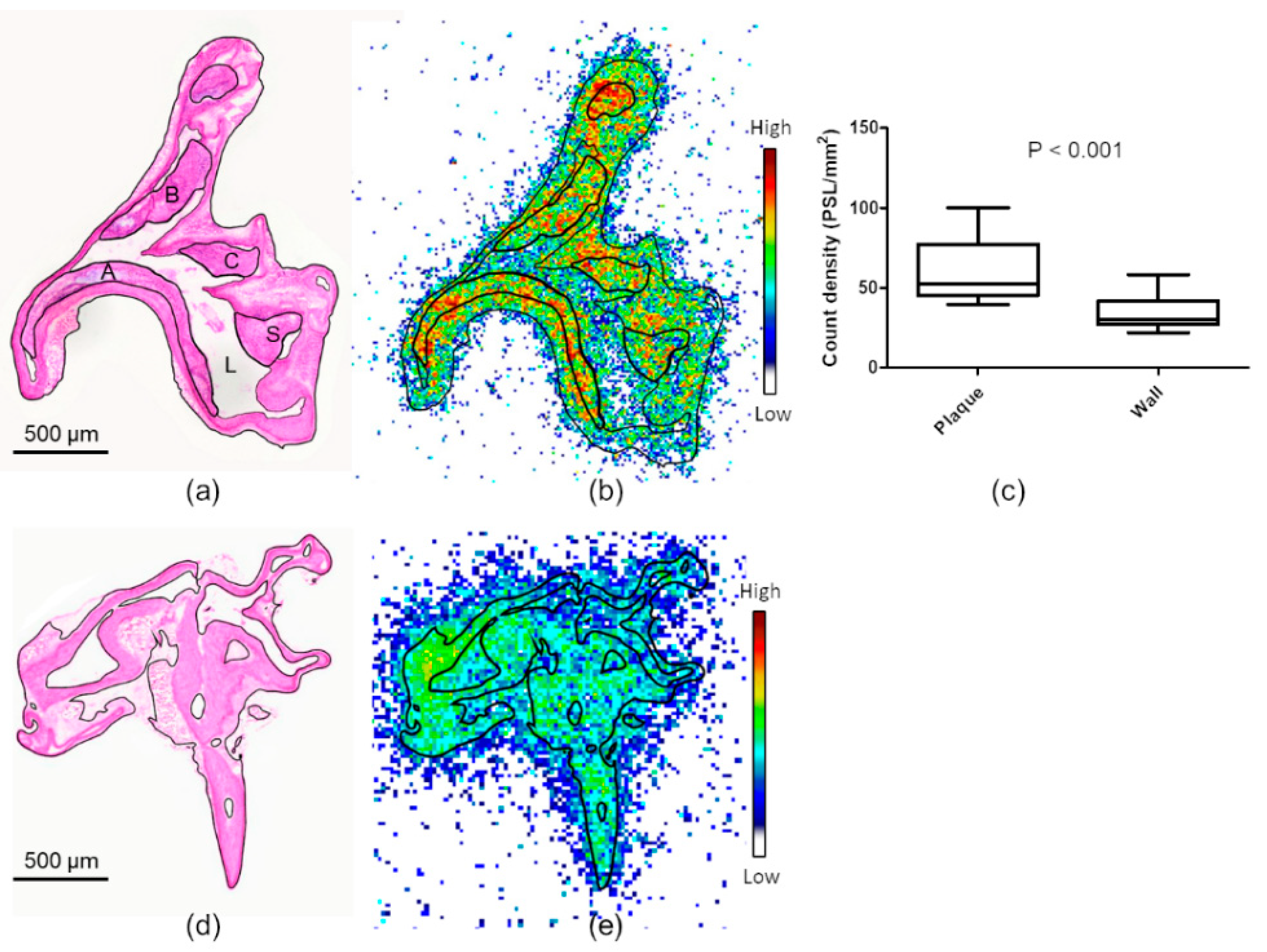
2.2. Ex Vivo Autoradiography and Biodistribution in Mice
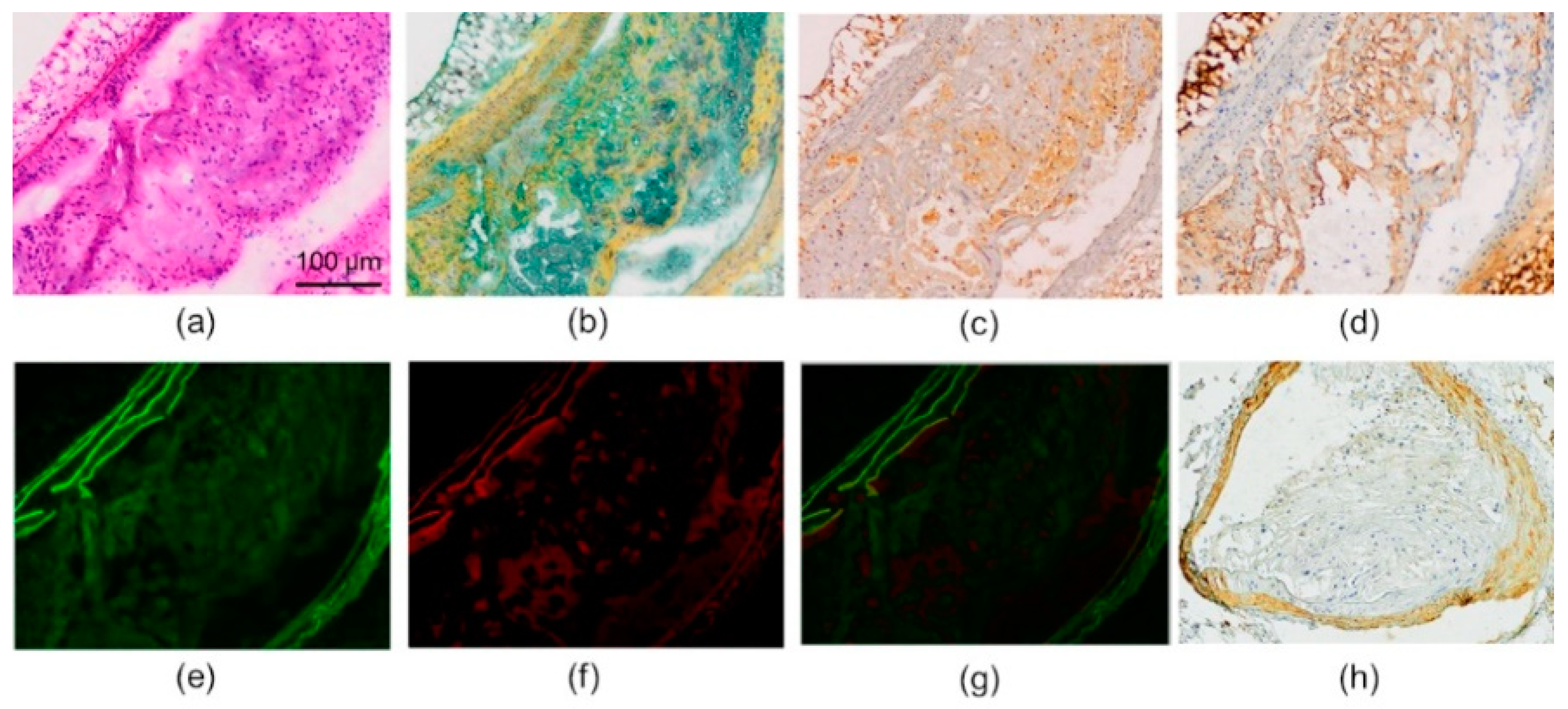
2.3. Histology and Immunohistochemistry
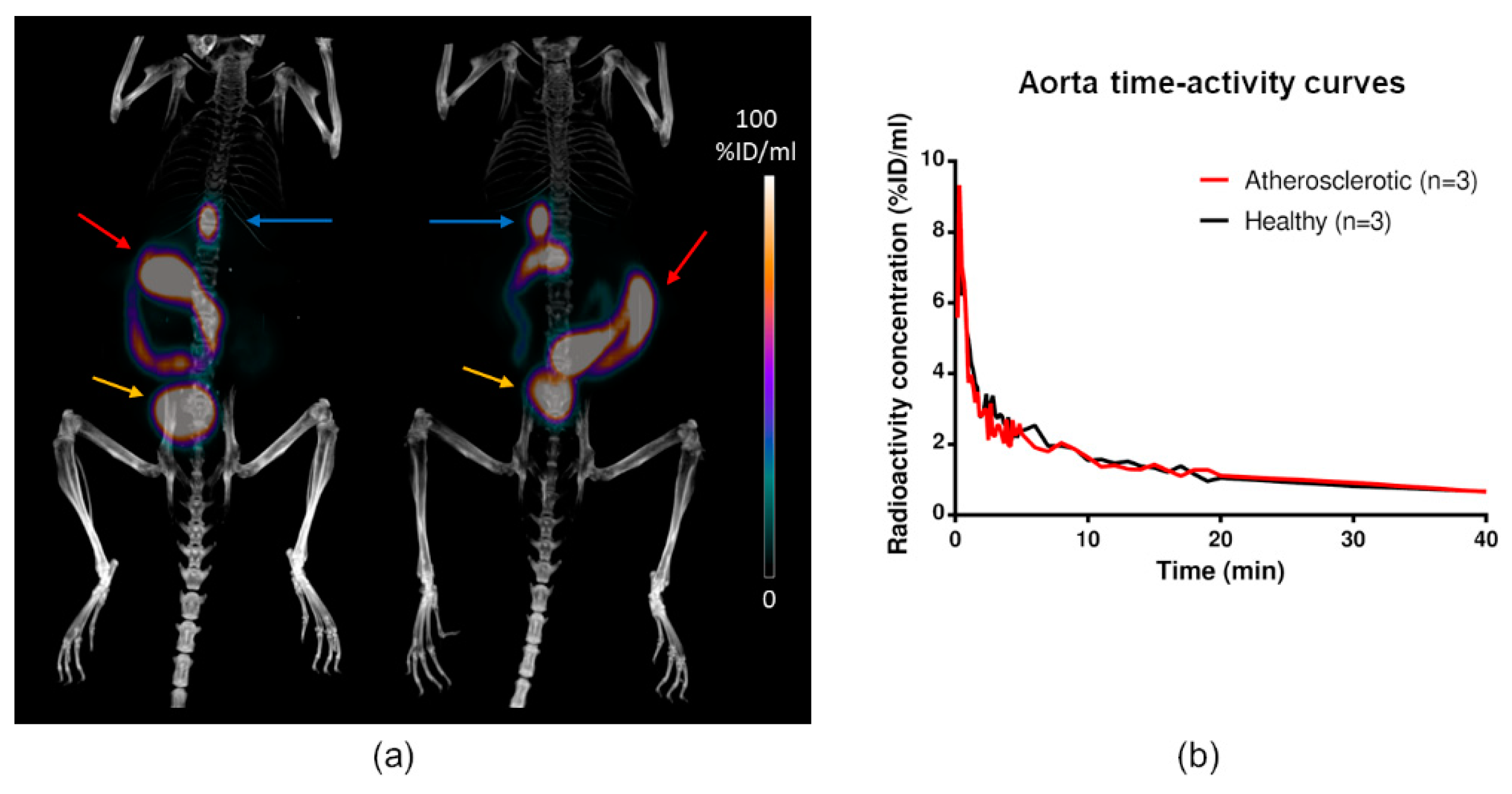
2.4. In Vivo PET/CT Imaging
2.5. Metabolite Analysis
3. Discussion
4. Materials and Methods
4.1. Radiochemistry
4.2. In Vitro Binding in Human Atherosclerotic Plaque
4.3. Animals
4.4. Ex Vivo Biodistribution and Aortic Autoradiography
4.5. Immunohistochemistry
4.6. In Vivo PET/CT Imaging
4.7. Radiometabolite Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in Atherosclerosis. From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Casserly, I.; Topol, E. Convergence of atherosclerosis and Alzheimer’s disease: Inflammation, cholesterol, and misfolded proteins. Lancet 2004, 363, 1139–1146. [Google Scholar] [CrossRef]
- Lathe, R.; Sapronova, A.; Kotelevtsev, Y. Atherosclerosis and Alzheimer—Diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr. 2014, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Martins, R.N.; Rumble, B.; Moir, R.; Fuller, S.; Milward, E.; Currie, D.; Ames, A. The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J. Biol. Chem. 1990, 265, 15977–15983. [Google Scholar] [PubMed]
- Kitazume, S.; Tachida, Y.; Kato, M.; Yamaguchi, Y.; Honda, T.; Hashimoto, Y.; Wada, Y.; Saito, T.; Iwata, N.; Saido, T.; et al. Brain endothelial cells produce amyloid β from amyloid precursor protein 770 and preferentially secrete the O-glycosylated form. J. Biol. Chem. 2010, 285, 40097–40103. [Google Scholar] [CrossRef] [PubMed]
- Sisodia, S.S. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc. Natl. Acad. Sci. USA 1992, 89, 6075–6079. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Yoshihisa, A.; Yamaki, T.; Oikawa, M.; Tachida, Y.; Ogawa, K.; Imamaki, R.; Hagiwara, Y.; Kinoshita, N.; Takeishi, Y.; et al. Soluble amyloid precursor protein 770 is released from inflamed endothelial cells and activated platelets: A novel biomarker for acute coronary syndrome. J. Biol. Chem. 2012, 287, 40817–40825. [Google Scholar] [CrossRef]
- Stamatelopoulos, K.; Sibbing, D.; Rallidis, L.S.; Georgiopoulos, G.; Stakos, D.; Braun, S.; Gatsiou, A.; Sopova, K.; Kotakos, C.; Varounis, C.; et al. Amyloid-Beta (1-40) and the Risk of Death from Cardiovascular Causes in Patients with Coronary Heart Disease. J. Am. Coll. Cardiol. 2015, 65, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D.; Kip, K.E.; Marroquin, O.C.; Ridker, P.M.; Kelsey, S.F.; Shaw, L.J.; Pepine, C.J.; Sharaf, B.; Merz, C.N.B.; Sopko, G.; et al. Serum Amyloid A as a Predictor of Coronary Artery Disease and Cardiovascular Outcome in Women: The National Heart; Lung; and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004, 109, 726–732. [Google Scholar] [CrossRef]
- Mucchiano, G.I.; Häggqvist, B.; Sletten, K.; Westermark, P. Apolipoprotein A-1-derived amyloid in atherosclerotic plaques of the human aorta. J. Pathol. 2001, 193, 270–275. [Google Scholar] [CrossRef]
- De Meyer, G.R.Y.; De Cleen, D.M.M.; Cooper, S.; Knaapen, M.W.M.; Jans, D.M.; Martinet, W.; Herman, A.G.; Bult, H.; Kockx, M.M. Platelet phagocytosis and processing of β-amyloid precursor protein as a mechanism of macrophage activation in atherosclerosis. Circ. Res. 2002, 90, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Kokjohn, T.A.; Van Vickle, G.D.; Maarouf, C.L.; Kalback, W.M.; Hunter, J.M.; Daugs, I.D.; Luehrs, D.C.; Lopez, J.; Brune, D.; Sue, L.I.; et al. Chemical characterization of pro-inflammatory amyloid-beta peptides in human atherosclerotic lesions and platelets. Biochim. Biophys. Acta 2011, 1812, 1508–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibolla, G.; Norata, G.D.; Meda, C.; Arnaboldi, L.; Uboldi, P.; Piazza, F.; Ferrarese, C.; Corsinia, A.; Maggia, A.; Vegetoa, E.; et al. Increased atherosclerosis and vascular inflammation in APP transgenic mice with apolipoprotein E deficiency. Atherosclerosis 2010, 210, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Van De Parre, T.J.L.; Guns, P.J.D.F.; Fransen, P.; Martinet, W.; Bult, H.; Herman, A.G.; De Meyer, G.R.Y. Attenuated atherogenesis in apolipoprotein E-deficient mice lacking amyloid precursor protein. Atherosclerosis 2011, 216, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Endemann, G.; Stanton, L.W.; Madden, K.S.; Bryant, C.M.; White, R.T.; Protter, A.A. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993, 268, 11811–18116. [Google Scholar] [PubMed]
- Febbraio, M.; Hajjar, D.P.; Silverstein, R.L. CD36: A class B scavenger receptor involved in angiogenesis; atherosclerosis; inflammation; and lipid metabolism. J. Clin. Investig. 2001, 108, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Tseng, A.A.; Mok, Y.F.; Staples, M.K.; Schiesser, C.H.; Lawrence, L.J.; Varghese, J.N.; Moore, K.J.; Howle, G.J. Oxidation of low-density lipoproteins induces amyloid-like structures that are recognized by macrophages. Biochemistry 2005, 44, 9108–9116. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging Brain Amyloid in Alzheimer’s Disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Herholz, K.; Ebmeier, K. Clinical amyloid imaging in Alzheimer’s disease. Lancet Neurol. 2011, 10, 667–670. [Google Scholar] [CrossRef]
- Bucerius, J.; Barthel, H.; Tiepolt, S.; Werner, P.; Sluimer, J.C.; Wildberger, J.E.; Patt, M.; Hesse, S.; Gertz, H.J.; Biessen, E.A.L.; et al. Feasibility of in vivo 18F-florbetaben PET/MR imaging of human carotid amyloid-β. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1119–1128. [Google Scholar] [CrossRef]
- Antoni, G.; Lubberink, M.; Estrada, S.; Axelsson, J.; Carlson, K.; Lindsjö, L.; Kero, T.; Långström, B.; Granstam, S.O.; Rosengren, S.; et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J. Nucl. Med. 2013, 54, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Vangala, D.; Semer, J.; Strader, C.; Bruyere, J.R.; Di Carli, M.F.; Moore, S.C.; Falk, R.H. Imaging cardiac amyloidosis: A pilot study using 18F-florbetapir positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, N.; Van Laere, K.; Thurfjell, L.; Owenius, R.; Vandenbulcke, M.; Koole, M.; Bormans, G.; Brooks, D.J.; Vandenberghe, R. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J. Nucl. Med. 2009, 50, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Bouma, B.; Kroon-Batenburg, L.M.J.; Wu, Y.P.; Brünjes, B.; Posthuma, G.; Kranenburg, O.; de Groot, P.G.; Voest, E.E.; Gebbink, M.F.B.G. Glycation Induces Formation of Amyloid Cross-β Structure in Albumin. J. Biol. Chem. 2003, 278, 41810–41819. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.E.; Leppänen, P.; Kholová, I.; Lumivuori, H.; Häkkinen, S.K.; Bosch, F.; Laakso, M.; Ylä-Herttuala, S. Increased atherosclerotic lesion calcification in a novel mouse model combining insulin resistance; hyperglycemia; and hypercholesterolemia. Circ. Res. 2007, 101, 1058–1067. [Google Scholar] [CrossRef]
- Tsimikas, S.; Shortal, B.P.; Witztum, J.L.; Palinski, W. In vivo uptake of radiolabeled MDA2; an oxidation-specific monoclonal antibody; provides an accurate measure of atherosclerotic lesions rich in oxidized LDL and is highly sensitive to their regression. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 689–697. [Google Scholar] [CrossRef]
- Nishigori, K.; Temma, T.; Yoda, K.; Onoe, S.; Kondo, N.; Shiomi, M.; Ono, M.; Saji, H. Radioiodinated peptide probe for selective detection of oxidized low density lipoprotein in atherosclerotic plaques. Nucl. Med. Biol. 2013, 40, 97–103. [Google Scholar] [CrossRef]
- Mucchiano, G.; Cornwell, G.G.; Westermark, P. Senile aortic amyloid. Evidence for two distinct forms of localized deposits. Am. J. Pathol. 1992, 140, 871–877. [Google Scholar]
- Klunk, W.E.; Lopresti, B.J.; Ikonomovic, M.D.; Lefterov, I.M.; Koldamova, R.P.; Abrahamson, E.E.; Debnath, M.L.; Holt, D.P.; Huang, G.; Shao, L.; et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer’s disease brain but not in transgenic mouse brain. J. Neurosci. 2005, 25, 10598–10606. [Google Scholar] [CrossRef]
- Adlard, P.A.; Tran, B.A.; Finkelstein, D.I.; Desmond, P.M.; Johnston, L.A.; Bush, A.I.; Egan, G.F. A review of β-amyloid neuroimaging in Alzheimer’s disease. Front. Neurosci. 2014, 8, 327. [Google Scholar] [CrossRef]
- Snellman, A.; Rokka, J.; Lopez-Picon, F.R.; Eskola, O.; Wilson, I.; Farrar, G.; Scheinin, M.; Solin, O.; Rinne, J.O.; Haaparanta-Solin, M. Pharmacokinetics of [18F]flutemetamol in wild-type rodents and its binding to beta amyloid deposits in a mouse model of Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Haukkala, J.; Laitinen, I.; Luoto, P.; Iveson, P.; Wilson, I.; Karlsen, H.; Cuthbertson, A.; Laine, J.; Leppänen, P.; Ylä-Herttula, S.; et al. 68Ga-DOTA-RGD peptide: Biodistribution and binding into atherosclerotic plaques in mice. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Lehti, S.; Käkelä, R.; Hörkkö, S.; Kummu, O.; Helske-Suihko, S.; Kupari, M.; Werkkala, K.; Kovanen, P.T.; Öörni, K. Modified Lipoprotein-Derived Lipid Particles Accumulate in Human Stenotic Aortic Valves. PLoS ONE 2013, 8, e65810. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| In Vivo PET (%ID/mL) | Ex Vivo Biodistribution (%ID/g) | |||||
|---|---|---|---|---|---|---|
| Tissue | Atherosclerotic (n = 3) | Control (n = 3) | p Value | Atherosclerotic (n = 10) | Control (n = 8) | p Value |
| Aorta | 0.87 ± 0.091 | 0.82 ± 0.10 | 0.55 | 0.81 ± 0.21 | 0.65 ± 0.20 | 0.12 |
| Blood | 1.0 ± 0.27 | 1.5 ± 0.043 | 0.15 | 1.0 ± 0.58 | 0.55 ± 0.21 | 0.055 |
| Brain | 1.2 ± 0.038 | 1.4 ± 0.36 | 0.55 | N/A | N/A | N/A |
| Gallbladder | 110 ± 32 | 97 ± 22 | 0.64 | N/A | N/A | N/A |
| Intestine | 78 ± 48 | 84 ± 19 | 0.89 | N/A | N/A | N/A |
| Kidneys | 9.8 ± 2.0 | 11 ± 3.7 | 0.62 | 16 ± 12 | 14 ± 4.4 | 0.65 |
| Liver | 4.8 ± 0.36 | 6.7 ± 0.65 | 0.032 | 4.6 ± 2.1 | 8.0 ± 5.2 | 0.074 |
| Muscle | 0.30 ± 0.032 | 0.32 ± 0.026 | 0.52 | 0.26 ± 0.092 | 0.29 ± 0.10 | 0.44 |
| Myocardium | 0.82 ± 0.0021 | 1.0 ± 0.12 | 0.13 | 0.49 ± 0.24 | 0.37 ± 0.18 | 0.25 |
| Urine | 69 ± 26 | 100 ± 28 | 0.30 | 240 ± 180 | 120 ± 65 | 0.081 |
| WAT | 0.56 ± 0.37 | 0.49 ± 0.22 | 0.83 | 0.48 ± 0.20 | 0.40 ± 0.32 | 0.51 |
| Tissue | Control (Male, n = 8) | Atherosclerotic (Male n = 5) | p Value (vs. Control) | Atherosclerotic (Female, n = 5) | p Value (vs. Control) | p Value (vs. Male Atherosclerotic) |
|---|---|---|---|---|---|---|
| Aorta | 0.65 ± 0.20 | 0.70 ± 0.11 | 0.60 | 0.92 ± 0.20 | 0.063 | 0.11 |
| Blood | 0.55 ± 0.21 | 0.59 ± 0.031 | 0.65 | 1.3 ± 0.53 | 0.045 | 0.055 |
| Kidney | 14 ± 4.4 | 9.1 ± 3.4 | 0.064 | 23 ± 12 | 0.21 | 0.084 |
| Liver | 8.0 ± 5.2 | 3.3 ± 0.88 | 0.038 | 5.8 ± 2.0 | 0.32 | 0.048 |
| Muscle | 0.29 ± 0.10 | 0.19 ± 0.023 | 0.024 | 0.32 ± 0.079 | 0.60 | 0.014 |
| Myocardium | 0.37 ± 0.18 | 0.33 ± 0.054 | 0.58 | 0.66 ± 0.21 | 0.057 | 0.036 |
| Plasma | 0.79 ± 0.24 | 1.0 ± 0.22 | 0.14 | 1.8 ± 0.82 | 0.11 | 0.10 |
| Urine | 120 ± 65 | 280 ± 180 | 0.15 | 190 ± 130 | 0.42 | 0.46 |
| WAT | 0.40 ± 0.32 | 0.41 ± 0.090 | 0.89 | 0.55 ± 0.23 | 0.38 | 0.32 |
| Biodistribution + ARG | In Vivo PET/CT | Metabolite Analysis | ||||
|---|---|---|---|---|---|---|
| Atherosclerotic | Control | Atherosclerotic | Control | Atherosclerotic | Control | |
| No. of animals (m/f) | 10 (5/5) | (8/0) | 3 (3/0) | 3 (3/0) | 3 (3/0) | 3 (3/0) |
| Age (months) | 6–8 | 7–11 | 8 | 3 | 9 | 7 |
| High-fat diet (months) | 4–5 | N/A | 4 | N/A | 5 | N/A |
| Weight (g) | 35 ± 7.0 | 44 ± 3.3 | 42 ± 3.4 | 33 ± 2.7 | 38 ± 6.3 | 37 ± 8.3 |
| Injected radioactivity (MBq) | 11 ± 0.95 | 11 ± 2.1 | 4.7 ± 1.1 | 5.4 ± 1.3 | 9.2 ± 1.3 | 11 ± 0.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellberg, S.; Silvola, J.M.U.; Liljenbäck, H.; Kiugel, M.; Eskola, O.; Hakovirta, H.; Hörkkö, S.; Morisson-Iveson, V.; Hirani, E.; Saukko, P.; et al. Amyloid-Targeting PET Tracer [18F]Flutemetamol Accumulates in Atherosclerotic Plaques. Molecules 2019, 24, 1072. https://doi.org/10.3390/molecules24061072
Hellberg S, Silvola JMU, Liljenbäck H, Kiugel M, Eskola O, Hakovirta H, Hörkkö S, Morisson-Iveson V, Hirani E, Saukko P, et al. Amyloid-Targeting PET Tracer [18F]Flutemetamol Accumulates in Atherosclerotic Plaques. Molecules. 2019; 24(6):1072. https://doi.org/10.3390/molecules24061072
Chicago/Turabian StyleHellberg, Sanna, Johanna M.U. Silvola, Heidi Liljenbäck, Max Kiugel, Olli Eskola, Harri Hakovirta, Sohvi Hörkkö, Veronique Morisson-Iveson, Ella Hirani, Pekka Saukko, and et al. 2019. "Amyloid-Targeting PET Tracer [18F]Flutemetamol Accumulates in Atherosclerotic Plaques" Molecules 24, no. 6: 1072. https://doi.org/10.3390/molecules24061072





