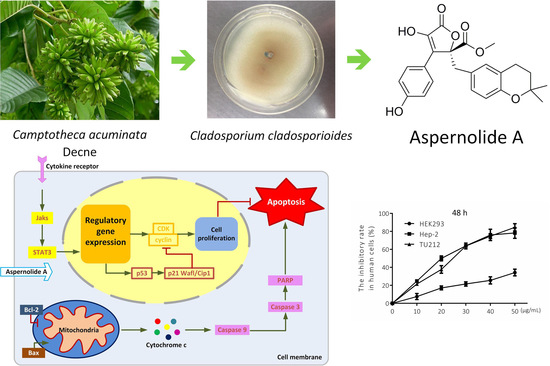
Aspernolide A Inhibits the Proliferation of Human Laryngeal Carcinoma Cells through the Mitochondrial Apoptotic and STAT3 Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Effects of Aspernolide A on Cell Viability in Hep-2 and TU212 Cells
2.2. Effects of Aspernolide A on Cell Migration and Colony Formation in Hep-2 and TU212 Cells
2.3. Effects of Aspernolide A on Morphological Changes and Apoptosis in Hep-2 and TU212 Cells
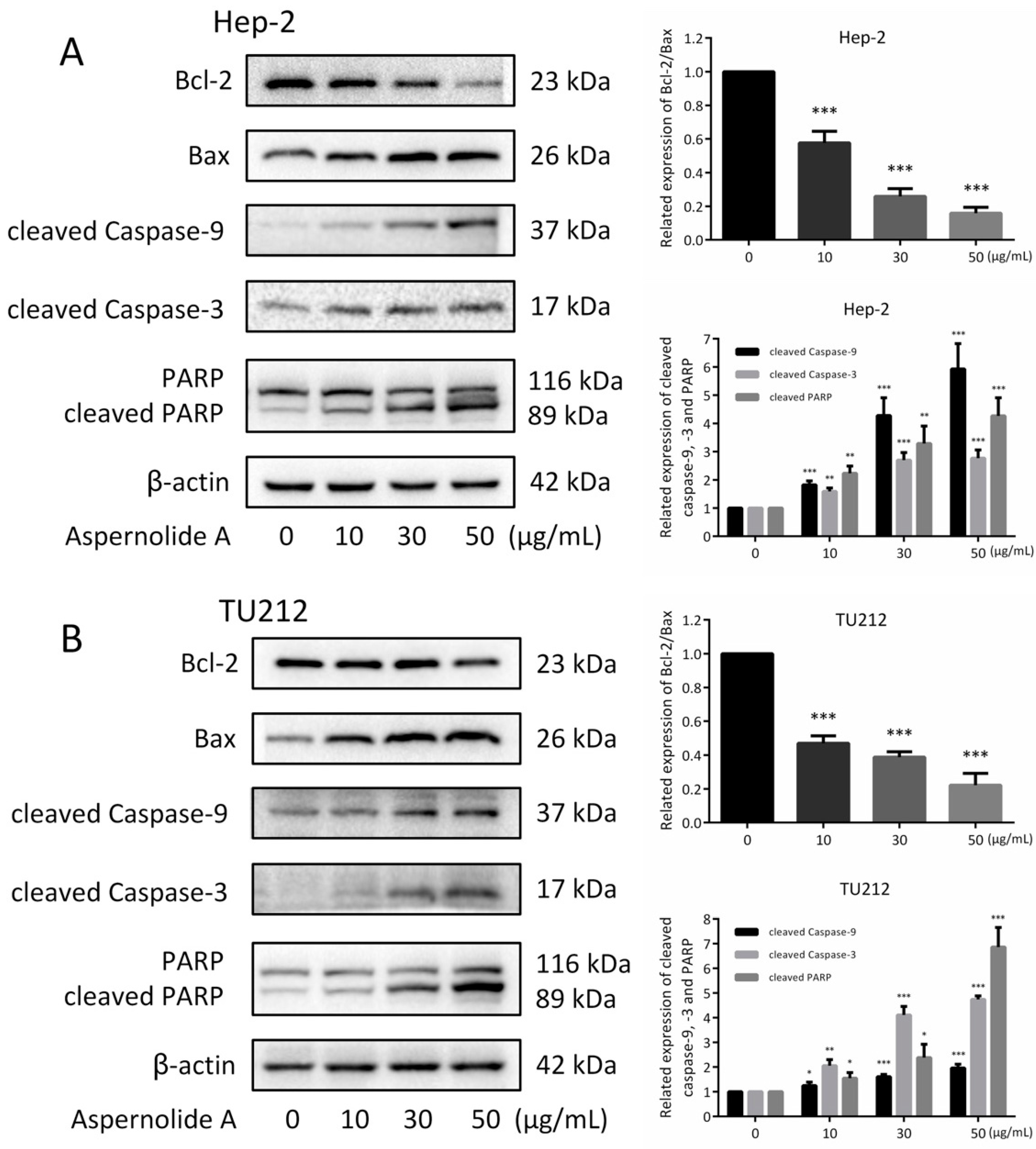
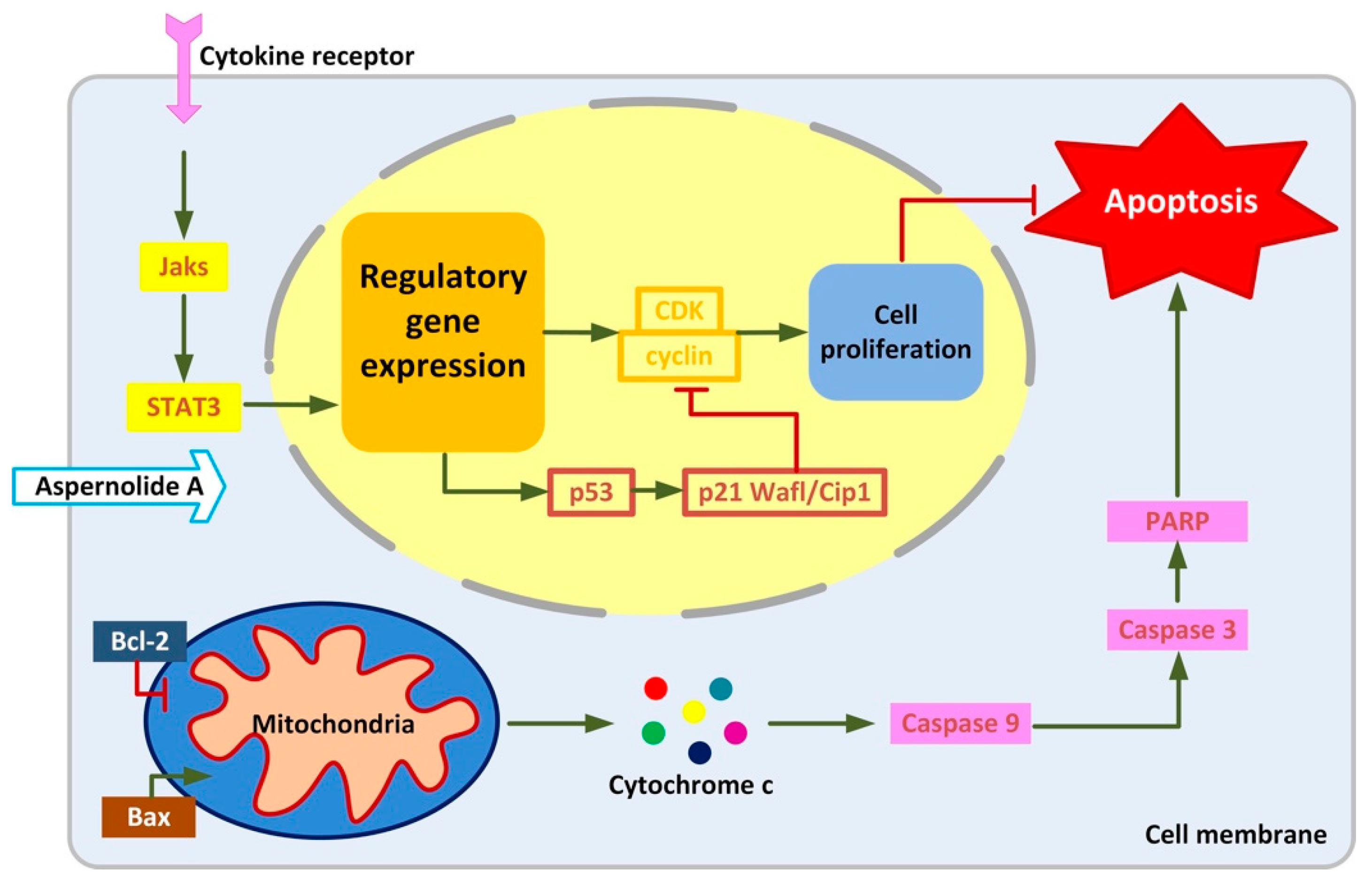
2.4. Effects of Aspernolide A on the Mitochondrial Apoptotic Pathway in Hep-2 and TU212 Cells
2.5. Effect of Aspernolides A on STAT3 Signaling Pathway in Hep-2 and TU212 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
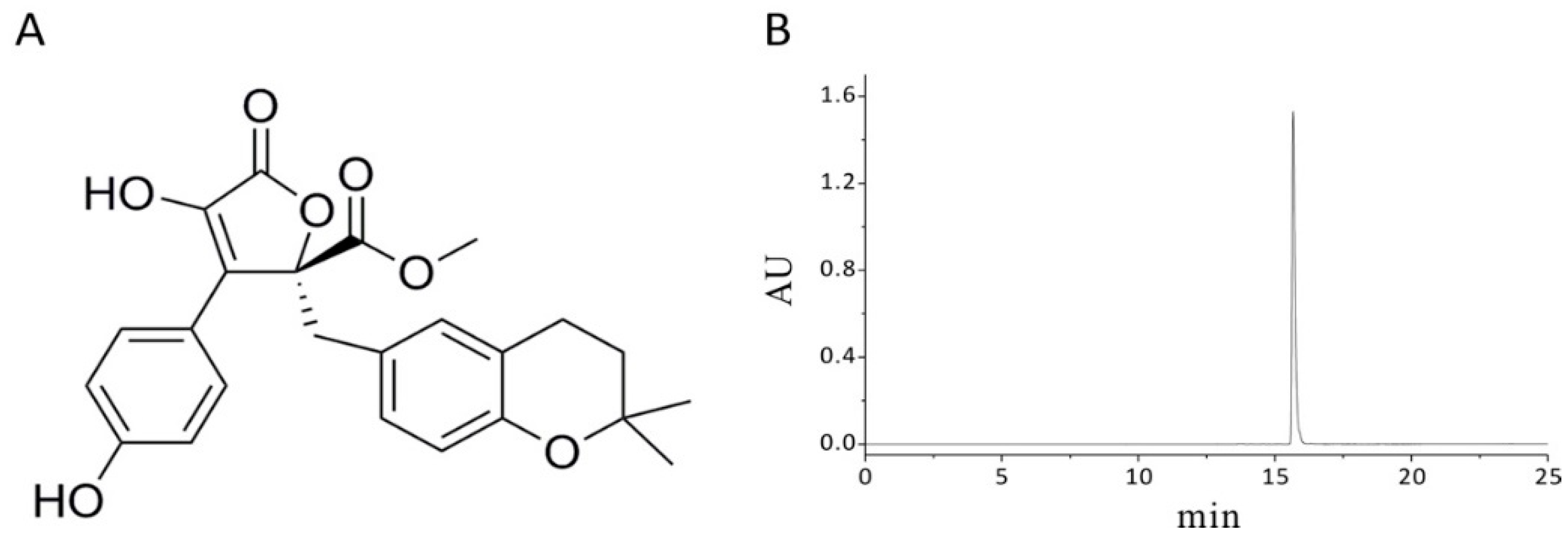
4.2. General Experimental Procedures
4.3. Source and Isolation of Fungus
4.4. Extraction and Isolation
4.5. Cell Culture
4.6. MTT Assay
4.7. Morphological Changes of Apoptosis
4.8. Scratch Assay
4.9. Colony Forming Test
4.10. Annexin V-FITC Double Staining
4.11. Western Blotting
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Riboli, E.; Kaaks, R.; Esteve, J. Nutrition and laryngeal cancer. Cancer Causes Control 1996, 7, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lionello, M.; Lovato, A.; Staffieri, A.; Blandamura, S.; Turato, C.; Giacomelli, L.; Staffieri, C.; Marioni, G. The EGFR-mTOR pathway and laryngeal cancer angiogenesis. Eur. Arch. Otorhinolaryngol. 2014, 271, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Maurizi, M.; Almadori, G.; Ferrandina, G.; Distefano, M.; Romanini, M.E.; Cadoni, G.; Benedetti-Panici, P.; Paludetti, G.; Scambia, G.; Mancuso, S. Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Br. J. Cancer 1996, 74, 1253–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Biswas, K.D.; Ghatak, S.; Haldar, D.; Sen, I.; Sinha, R. Postoperative hypofunctioning of the thyroid gland after total laryngectomy. Ear Nose Throat J. 2016, 95, E23–E27. [Google Scholar] [PubMed]
- Liu, M.; Wu, H.; Liu, T.; Li, Y.; Wang, F.; Wan, H.; Li, X.; Tang, H. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res. 2009, 19, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, F.; Ishibashi, M. Bio-active Natural Products with TRAIL-Resistance Overcoming Activity. Chem. Pharm. Bull. 2016, 64, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Rivory, L.P.; Robert, J. Molecular, cellular, and clinical aspects of the pharmacology of 20(S)camptothecin and its derivatives. Pharmacol. Ther 1995, 68, 269–296. [Google Scholar] [CrossRef]
- Cragg, G.M. Paclitaxel (Taxol): A success story with valuable lessons for natural product drug discovery and development. Med. Res. Rev. 1998, 18, 315–331. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Yoshida, K.; Abe, N.; Hirota, A. Soybean lipoxygenase inhibitory and DPPH radical-scavenging activities of aspernolide A and butyrolactones I and II. Biosci. Biotechnol. Biochem. 2010, 74, 881–883. [Google Scholar] [CrossRef]
- Parvatkar, R.R.; D’Souza, C.; Tripathi, A.; Naik, C.G. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry 2009, 70, 128–132. [Google Scholar] [CrossRef]
- Zhao, N.; Tian, K.T.; Cheng, K.G.; Han, T.; Hu, X.; Li, D.H.; Li, Z.L.; Hua, H.M. Antiproliferative activity and apoptosis inducing effects of nitric oxide donating derivatives of evodiamine. Bioorg. Med. Chem. 2016, 24, 2971–2978. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Ma, Y.; Wright, G.M.; Huang, M.; Irby, R.; Briggs, J.; Karras, J.; Cress, W.D.; Pardoll, D.; et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell Biol. 2005, 25, 7432–7440. [Google Scholar] [CrossRef]
- Narayanan, B.A.; Geoffroy, O.; Willingham, M.C.; Re, G.G.; Nixon, D.W. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999, 136, 215–221. [Google Scholar] [CrossRef]
- Hong, H.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Kanagawa, O.; Nakagawa, M.; Okita, K.; Yamanaka, S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 2009, 460, 1132–1135. [Google Scholar] [CrossRef]
- Almadori, G.; Bussu, F.; Cadoni, G.; Galli, J.; Paludetti, G.; Maurizi, M. Molecular markers in laryngeal squamous cell carcinoma: Towards an integrated clinicobiological approach. Eur. J. Cancer 2005, 41, 683–693. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Chin, Y.W.; Swanson, S.M. Discovery of natural product anticancer agents from biodiverse organisms. Curr. Opin. Drug Discov. Devel. 2009, 12, 189–196. [Google Scholar]
- Jain, S.K.; Pathania, A.S.; Parshad, R.; Raina, C.; Ali, A.; Gupta, A.P.; Kushwaha, M.; Aravinda, S.; Bhushan, S.; Bharate, S.B.; et al. Chrysomycins A–C, antileukemic naphthocoumarins from Streptomyces sporoverrucosus. RSC Adv. 2013, 3, 21046. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Zhou, Y.T.; Levi, M.; Unger, R.H. Fatty acid-induced β cell apoptosis: A link between obesity and diabetes. Proc. Natl. Acad. Sci. USA 1998, 95, 2498–2502. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Porter, K.; Parameswaran, N.; Bae, H.K.; Pestka, J.J. Role of GRP78/BiP degradation and ER stress in deoxynivalenol-induced interleukin-6 upregulation in the macrophage. Toxicol. Sci. 2009, 109, 247–255. [Google Scholar] [CrossRef]
- Hickman, J.A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992, 11, 121–139. [Google Scholar] [CrossRef]
- Zhai, Z.H.; Wang, X.Z.; Ding, M.X. Cell Biology; Higher Education Press: Beijing, China, 2011; pp. 313–314. [Google Scholar]
- Yang, J.; Xu, C.; Chen, H.; Huang, M.; Ma, X.H.; Deng, S.H.; Huang, Y.; Wen, Y.Z.; Yang, X.Z.; Song, P. In vitro and in vivo antitumor effects of the diterpene-enriched extract from Taxodium ascendens through the mitochondrial-dependent apoptosis pathway. Biomed. Pharmacother. 2017, 96, 1199–1208. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Se-Kwon, K. Cell survival and apoptosis signaling as therapeutic target for cancer: Marine bioactive compounds. Int. J. Mol. Sci. 2013, 14, 2334–2354. [Google Scholar] [CrossRef]
- Shoshanbarmatz, V.; Benhail, D. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion 2012, 12, 24–34. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Xing, D. Cell death via mitochondrial apoptotic pathway due to activation of Bax by lysosomal photodamage. Free Radic. Biol. Med. 2011, 51, 53–68. [Google Scholar] [CrossRef]
- Wen, H.; Zhou, S.; Song, J. Induction of apoptosis by magnolol via the mitochondrial pathway and cell cycle arrest in renal carcinoma cells. Biochem. Biophys. Res. Commun. 2019, 508, 1271–1278. [Google Scholar] [CrossRef]
- Leonard, W.J. Role of Jak kinases and STATs in cytokine signal transduction. Int. J. Hematol. 2001, 73, 271–277. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Chen, Y.; Lu, X. Expression of STAT3 and P-STAT3 in laryngeal carcinoma and its clinical significance. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2007, 21, 113–115. [Google Scholar]
- Chen, H.; Zhou, B.; Yang, J.; Ma, X.H.; Deng, S.H.; Huang, Y.; Wen, Y.Z.; Yuan, J.Q.; Yang, X.Z. Essential Oil Derived from Eupatorium adenophorum Spreng. Mediates Anticancer Effect by Inhibiting STAT3 and AKT Activationto Induce Apoptosis in Hepatocellular Carcinoma. Front. Pharmacol. 2018, 9, 483. [Google Scholar] [CrossRef]
- Chen, X.; Bargonetti, J.; Prives, C. p53, through p21 (WAF1/CIP1), Induces Cyclin D1 Synthesis. Cancer Res. 1995, 55, 4257–4263. [Google Scholar] [CrossRef]
- Dai, B.; Shi, X.; Ma, N.; Ma, W.; Zhang, Y.; Yang, T.; Zhang, J.; He, L. HMQ-T-B10 induces human liver cell apoptosis by competitively targeting EphrinB2 and regulating its pathway. J. Cell. Mol. Med. 2018, 22, 5231–5243. [Google Scholar] [CrossRef]
- Lin, L.; Deng, W.; Tian, Y.; Chen, W.; Wang, J.; Fu, L.; Shi, D.; Zhao, M.; Luo, W. Lasiodin inhibits proliferation of human nasopharyngeal carcinoma cells by simultaneous modulation of the Apaf-1/caspase, AKT/MAPK and COX-2/NF-kappaB signaling pathways. PLoS ONE 2014, 9, e97799. [Google Scholar] [CrossRef]
- Ma, X.H.; Yang, J.; Brown, C.L.; Wang, C.; Deng, S.H.; Ke, R.F.; Xu, S.C.; Huang, M.; Yang, X.Z.; Feng, Y.J. Cytotoxic neolignans from the traditional Chinese medicine Daphniphyllum macropodum Miq. RSC Adv. 2017, 7, 52970–52976. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound is available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, H.; Wen, Y.; Huang, H.; Hao, J.; Lv, Y.; Qin, R.; Yang, X. Aspernolide A Inhibits the Proliferation of Human Laryngeal Carcinoma Cells through the Mitochondrial Apoptotic and STAT3 Signaling Pathways. Molecules 2019, 24, 1074. https://doi.org/10.3390/molecules24061074
Liu C, Liu H, Wen Y, Huang H, Hao J, Lv Y, Qin R, Yang X. Aspernolide A Inhibits the Proliferation of Human Laryngeal Carcinoma Cells through the Mitochondrial Apoptotic and STAT3 Signaling Pathways. Molecules. 2019; 24(6):1074. https://doi.org/10.3390/molecules24061074
Chicago/Turabian StyleLiu, Chang, Hong Liu, Yanzhang Wen, Huiqi Huang, Ji Hao, Yibing Lv, Rui Qin, and Xinzhou Yang. 2019. "Aspernolide A Inhibits the Proliferation of Human Laryngeal Carcinoma Cells through the Mitochondrial Apoptotic and STAT3 Signaling Pathways" Molecules 24, no. 6: 1074. https://doi.org/10.3390/molecules24061074